FY2015 Regulatory Science Research Report: Nanotechnology: Physiochemical Characterization of Nano-Sized Drug Products
Introduction
Nano-sized drug products, such as nanoemulsions, protein-drug complexes and iron colloids, are complex drug products that require comprehensive physicochemical characterization to gain insight into product quality that can eventually affect in vivo performance. For many generic nano-products, equivalence in physicochemical properties (such as particle morphology, surface potential, size distribution, carrier composition, and state of the encapsulated drug) must be demonstrated. In many instances, characterization of these products is challenging due to the inherent complexity of the formulation. Additionally, the test product should possess a drug release profile that compares to the profile of the reference product, since bioavailability can be markedly influenced by differences in drug release from the formulations. Despite the increasing number of nano-sized products on the market, compendial or bio-relevant in vitro drug release assays for these complex dosage forms are in short supply. This shortage, along with complexities in physicochemical properties, poses a significant challenge to science-based policy development as well as the regulatory review of this product category.
Research
A collaborative research program has been established with the Office of Science and Engineering Laboratories at the Center for Devices and Radiological Health (CDRH) through which physicochemical characterization of several nanomaterials and liposomal products are being routinely conducted. Collaborative projects in this area will advance our understanding of the use of nanotechnology in developing generic drug products.
Figure 1. Negative-staining TEM and Cryo-TEM images of Restasis®
Negative-staining TEM (left) and Cryo-TEM (right) image of Restasis®, taken as part of physicochemical characterization studies at CDRH.
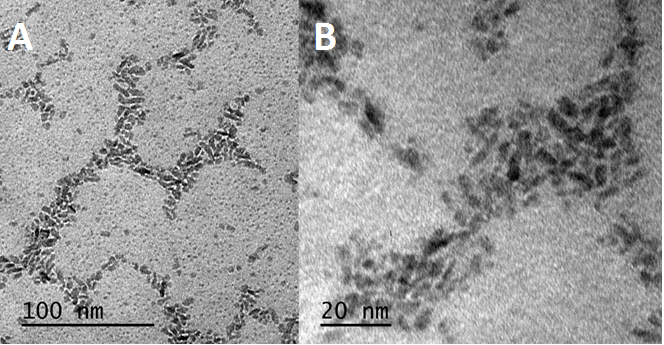
Figure 2. TEM image of INFeD showing elongated shape of individual particles
ORS staff facilitating research in this area
- Mohammad Absar, Stephanie Choi, and Darby Kozak
Projects and Collaborators
- Internal Project: Characterization of Nano-Sized Generic Drug Products
- FDA Collaborator: Jiwen Zheng
- FDA Center/Office/Division: CDRH/OSEL/DBCMS
Publications and Presentations
- Characterization of the globule size distribution of Cyclosporine ophthalmic emulsion to establish bioequivalence of a Test formulation. Peter E. Petrochenko, Mohammad S. Absar, Yong Wu, Sook Y. Wong, Stephanie Choi, Jiwen Zheng. AAPS Orlando, FL (October 25-29, 2015)
- Establishing Optimal Characterization Methods for Nanosized Generic and Brand Drug Comparison. Peter E. Petrochenko, Lynn Chen, Stephanie Choi, Mohammad S. Absar, Jiwen Zheng. FDA Science Forum (2015)
- Particle size characterization of iron sucrose colloids to study equivalence between test and reference products. Mohammad S. Absar, Nariman Ziaee, Lynn Chen, Stephanie Choi, Jiwen Zheng. AAPS Orlando, FL (October 25-29, 2015)
Outcomes
- Revision of Product-Specific Recommendation on Iron Sucrose Injection
- Product-Specific Recommendation on Iron Dextran Injection
- Revision of Product-Specific Recommendation on Cyclosporine Ophthalmic Emulsion
- Product-Specific Recommendation on Difluprednate Ophthalmic Emulsion

