FY2015 Regulatory Science Research Report: Locally-Acting Gastrointestinal Drugs
Introduction
Demonstrating BE for locally-acting GI drug products is challenging because these drug products reach the site of action before they enter systemic circulation. This category includes drugs such as prodrugs of mesalamine (sulfasalazine, balsalazide, olsalazine); controlled release mesalamine (Asacol, Pentasa, Lialda, Apriso); locally-acting steroids (budesonide); lubiprostone; binding and protective agents (acarbose, cholestyramine, sucralfate, orlistat, lanthanum carbonate, calcium acetate, sevelamer hydrochloride, sevelamer carbonate, colestipol HCl, colesevelam hydrochloride); locally-acting antibiotics (vancomycin, rifaximin, nitazoxanide, fidaxomicin); and agents that modify GI motility (loperamide).
Research
Bioequivalence studies with clinical endpoints are generally insensitive to detecting formulation differences and require a large number of subjects. FDA has recommended a wide variety of BE tests for these products, including an in vitro binding assay for cholestyramine, biowaivers for IR high solubility drugs, PK studies for mesalamine prodrugs and loperamide, and clinical equivalence studies for other products. The selection of the BE method is based on product-specific factors and a scientific understanding of the product’s mechanism of action. To develop alternative approaches for demonstrating BE for locally-acting GI drug products, FDA has taken an integrated approach by combining physiologically-based absorption (PBA) modeling, in vitro dissolution testing, and in vivo studies.
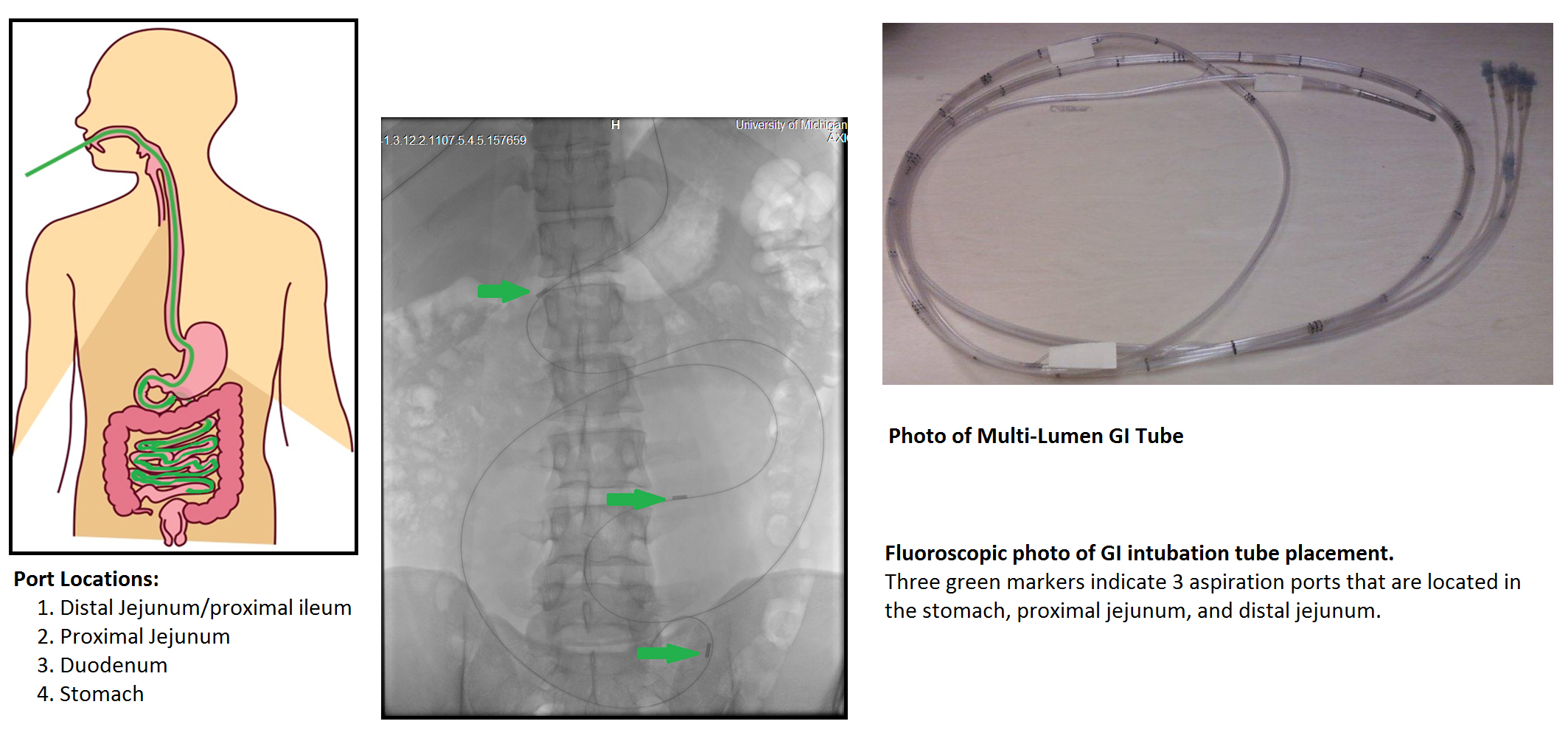
In the study “Correlation of Mesalamine Pharmacokinetics with Local Availability,” GI and plasma concentrations of mesalamine after oral administration of three mesalamine MR formulations were obtained. An IR arm was administered for PK deconvolution. The new GI local in vivo data combined with the systemic PK data supports expansion of PK and dissolution approaches for demonstration of BE to more locally-acting GI drug products such as lubiprostone, budesonide, and loperamide.
Another class of locally-acting GI drug products are binding agents that interfere with absorption of specific molecules. Collaborations with FDA laboratories have led to in vitro BE methods in this category, such as lanthanum carbonate products. Recent work has guided the essential material characterization for locally-acting GI products of complicated drug substances, such as sevelamer products.
Figure 13. Correlation of mesalamine pharmacokinetics with local availability
Source: Sun D. et al. Correlation of mesalamine pharmacokinetics with local availability, Annual Report (2014)
ORS staff facilitating research in this area
- Xinyuan Zhang, Hong Wen, Xiaohui Jiang, Deyi Zhang, and other ORS staff members
Projects and Collaborators
- Correlation of mesalamine pharmacokinetics with local availability
- Site PI: Duxin Sun, Ph. D.
- Contract #: HHSF223201300460A
Publications and Presentations
- Brown et al. A novel method to investigate local concentrations of mesalamine in the gastrointestinal tract of healthy volunteers. Digestive Disease Week, Chicago, Illinois (2014)
- Fioritto et al. Contrasting regional availability of different mesalamine products in the gastrointestinal tract in healthy humans using a novel multi-port luminal aspiration system and correlation with systemic absorption. Digestive Disease Week, Washington DC (2015)
- Yu et al. Direct Measurement of Mesalamine Dissolution in Human Gastrointestinal Tract. AAPS (2015)
Outcomes
- Draft bioequivalence guidance for mesalamine modified release oral products (6 products)
- Draft bioequivalence guidance for budesonide modified release oral products (2 products)
- Draft bioequivalence guidance for binding agents (calcium acetate tablets and capsules, cholestyramine oral powder, colesevelam hydrochloride powder for suspension, colesevelam hydrochloride tablet, ferric citrate tablets, lanthanum carbonate tablets and power, sevelamer carbonate tablets and suspension, sevelamer hydrochloride tablets, and sucroferric oxyhydroxide chewable tablets)
- Draft bioequivalence guidance for lubiprostone oral capsules