FY2013-2017 Regulatory Science Report: Oral Abuse-deterrent Opioid Products
Printer Friendly Version (PDF - 585 KB)
Introduction
Accomplishments (2012-2017)
Research and Collaboration
Key Outcomes
Future Directions
Outcomes
Introduction
Abuse and misuse of opioids has created a serious and widespread public health problem. The Centers for Disease Control and Prevention (CDC) estimates more than 33,000 overdose deaths were associated with prescription opioids and heroin in 2015. Regulatory agencies have made continuous efforts to reduce the prevalence of opioid abuse and addiction. One of the Food and Drug Administration (FDA) Commissioner Scott Gottlieb’s highest priorities is to reduce the scope of the epidemic of opioid addiction. One way that FDA is addressing this issue is encouraging the development of innovative abuse-deterrent (AD) oral solid dosage forms that are harder to abuse.
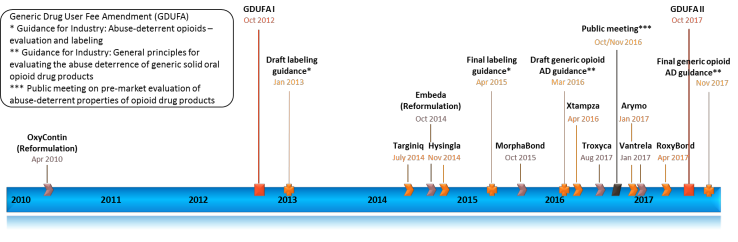
Between 2010 and 2017, FDA approved 10 brand-name oral opioids with labeling describing AD properties (Figure 1), starting with the approval of reformulated OxyContin (oxycodone hydrochloride extended-release (ER) tablet). There is still a knowledge gap in the scientific understanding pertaining to optimizing regulatory assessment of abuse-deterrent formulations (ADF) in the development pipeline. For generic drugs, the key regulatory challenge in abuse deterrence evaluation is the need to develop rigorous, meaningful and reliable measures to determine “non-inferior abuse deterrence” of generic versions of proprietary ADF.
Figure 1: Timeline of approval of brand-name oral ADF opioid products and selected FDA activities addressing development of generic opioid ADF
FDA has taken steps to respond to the increase in opioid-related deaths by initiating labeling changes to strengthen warnings about the abuse potential and requiring safety measures through Risk Evaluation and Mitigation Strategies (REMS) for ER and long-acting opioid products, as well as immediate-release opioids.
ADFs frequently require complex formulation designs and difficult scale-up/manufacturing processes. As of 2017, reformulated or new ADF opioid products are available only as brand-name products, which are often more expensive than the non-ADF generic products. Availability of generic ADF opioid products can help ensure patient access to affordable prescriptions. FDA leadership has emphasized the importance of generic ADF opioid products, indicating that, “the availability of less costly generic products should accelerate prescribers’ uptake of abuse-deterrent formulations.”
Accomplishments (2012-2017)
In 2013, FDA published the draft guidance Abuse-Deterrent Opioids – Evaluation and Labeling, which describes seven categories of AD technologies (physical/chemical barrier, agonist/antagonist combination, aversion, delivery system, new molecular entities/prodrug, combination, and novel approach). In most cases, to obtain a full and scientifically rigorous understanding of the impact of a technology on a product’s abuse potential, data from each of three categories of premarket studies should be provided to FDA in a new drug application (NDA):
- Laboratory-based in vitro manipulation and extraction studies
- Pharmacokinetic (PK) studies
- Clinical abuse potential studies (i.e., pharmacodynamics (PD) studies)
Based on review of approved innovator ADFs and research conducted by FDA, the Office of Generic Drugs (OGD) developed recommendations for the studies an abbreviated new drug application (ANDA) applicant should conduct and submit to FDA to demonstrate that a generic opioid intended to duplicate a brand name opioid with labeling describing abuse-deterrent properties is no less abuse deterrent than the reference listed drug (RLD) with respect to all potential routes of abuse. In March 2016, the draft guidance General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products was published. In November 2017, FDA finalized this guidance. Evaluation of generic ADFs involves a comprehensive and sequential approach including physical and chemical manipulation, in vitro evaluation, in vivo PK studies, and, in some instances, human abuse potential studies (i.e., PD studies).
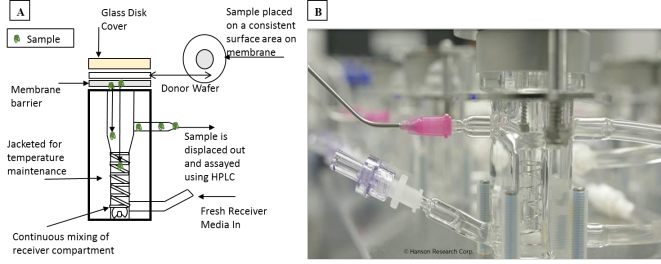
FDA’s guidance General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products describes physical manipulations (e.g., cutting, grating, milling) that should be used to gain an understanding of the robustness of abuse-deterrent properties. FDA conducted research to investigate the sensitivity of quantitative metrics that reflect the degree of difficulty of the manipulation methods employed, the extent to which each manipulation method succeeds in defeating the ADF mechanism (e.g., extractability), and the resulting quality attributes (e.g., particle size). For instance, a research contract at Purdue University examined different methods that people who abuse opioids use to circumvent AD properties of AD oxycodone hydrochloride ER tablets. The results led investigators to recommend that FDA adopt the studied modes of abuse as standardized methods of physical manipulations that are clinically relevant to various routes of abuse (e.g., oral, parenteral, nasal). The investigators also proposed an innovative test method employing the vertical diffusion cell (VDC) apparatus for the in vitro assessment of comminuted ADF prepared for the nasal route of abuse (Figure 2).
Figure 2: Schematic of the vertical diffusion cell (VDC) apparatus (A) and a side view of the Hanson VDC (B) used in the in vitro assessment of abuse deterrence (Image B was provided by Hanson Research Corp. and reproduced with their permission)
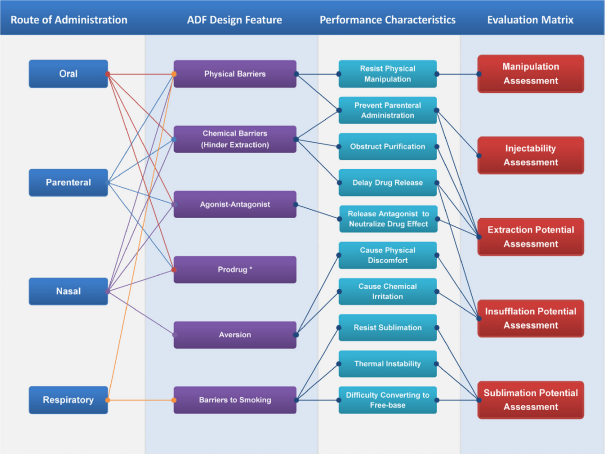
OGD and the Office of Pharmaceutical Quality (OPQ)’s Office of Testing and Research (OTR) collaborated on research designed to develop standard in vitro testing methods to assess the abuse deterrence of ADFs in an ANDA. The research helped FDA better understand AD properties and identify the strengths and failure modes of the reference ADF products; develop suitable in vitro methods comparing abuse-deterrent properties between the reference and test ADF products; and evaluate formulation and process variability and their potential impact on abuse-deterrent properties (Figure 3). This research contributed to the establishment of comparative in vitro methods for evaluating the potential for abuse between the generic ADF and the RLD.
Figure 3: Relationship between route of administration, ADF design features, product performance characteristics and matrices for evaluation.
*Evaluation of the performance of prodrug should consider the mechanism and rate of the conversion under various conditions, with permission of Elsevier.
In another study, OGD and OPQ’s OTR developed an in vitro chewing method intended to predict oral bioavailability of ADF opioid products following chewing. The simulated mastication method (using an Erweka DRT chewing machine (Figure 4) coupled with a United States Pharmacopeia (USP) dissolution apparatus) was optimized based on in-house PK data in the hope of developing an in vitro-in vivo correlation (IVIVC) using a physiologically-based pharmacokinetic (PBPK) absorption model. The goal of developing an in vitro chewing method is to assess AD performance of AD opioid products against chewing without the need for in vivo studies.
Figure 4: Images of Erweka DRT chewing machine (a) during the chewing cycles and (b) at the end of chewing study (Study report from the OPQ/ OTR)
Equally important in the evaluation of generic ADFs is comparison of the systemic exposure of opioids via in vivo PK studies. Comparing AD properties between reference and generic AD products via the nasal route requires evidence of reduced bioavailability and/or reduced human abuse potential after snorting of physically manipulated ADF opioid products. For this reason, a GDUFA-funded study investigated the effects of particle size and drug-to-polymer ratio on the bioavailability of milled AD OxyContin tablets following intranasal insufflation. The research project includes a four-treatment, single-dose, crossover clinical study that evaluates the PK and safety of milled oxycodone hydrochloride ER and immediate-release tablets following nasal insufflation in recreational opioid users. The results will be used to recommend an appropriate range of particle size of milled AD oxycodone hydrochloride ER tablets for comparative nasal PK studies.
Modeling and simulation (M&S) is an important tool guiding the design of in vivo studies used in generic drug research and development. OGD has been actively investigating the use of M&S in designing studies to evaluate abuse deterrence. For example, OGD, in collaboration with OPQ’s OTR, is constructing a PBPK absorption model to predict the nasal absorption of milled OxyContin tablets using in vitro dissolution and diffusion data (VDC apparatus and USP IV flow-through cell dissolution apparatus). The PBPK model will be validated by the computational fluid dynamics (CFD) analysis of regional deposition of milled particles in a respiratory model, similar to the framework established by Rygg and Longest7 for the evaluation of inhaled nasal corticosteroids (Figure 5). OGD is also investigating/considering the use of M&S to design studies to characterize the PK/PD relationship of ADF opioid products. In this quantitative modeling analysis, the preliminary results demonstrate that partial area-under-the-curve (pAUC) may be required in addition to conventional PK metrics (e.g., Cmax, AUCt and AUCinf) to conclude that a generic ADF opioid product is no less abuse-deterrent than its reference product.
Figure 5: Computational fluid dynamics (CFD) analysis (left) and 3-D printed respiratory model for fraction quantification of regional deposition (right) for in silico modeling of nasal absorption of milled OxyContin tablets (Image on the right provided by Dr. Ross Walenga)
Research Projects and Collaboration
Extramural Projects
- Evaluation of drug product formulation in vitro performance characteristics related to abuse deterrence for solid oral dosage forms of opioids
FDA awarded a contract to Stephen Byrn at Purdue University and Stephen Hoag at the University of Maryland on September 16, 2013, and the study was completed in September 2015. The goal of this contract was to examine different methods that abusers use to circumvent ADF oxycodone hydrochloride ER tablets and how different modes of abuse affect the dosage forms and their drug release rates. The study results showed that the FDA should account for different modes of abuse and have standardized methods for assessing each mode of abuse. A standardized test method for each route of abuse should predict how the abused product will interact with the body and the results of the testing should be submitted to FDA to support its evaluation of the generic drug.
- PK study of opioid drug product following insufflation of milled drug products
FDA awarded a contract to Bradley Vince at Vince and Associates on September 17, 2015, and the study is ongoing. The objective of this study is to investigate the effect of particle size and drug-to-polymer ratio that influence bioavailability of milled ADF oxycodone hydrochloride ER tablets following intranasal insufflation. A four-treatment, single-dose crossover study will be conducted to evaluate the PK and safety of milled ER oxycodone and milled immediate-release oxycodone following intranasal insufflation in recreational opioid users. The results will then be used to recommend appropriate particle sizes for the comparison of the generic to brand products in clinical studies in ANDAs.
Internal Projects
Development of in vitro methods evaluating abuse deterrence of ADFs in support of developing FDA’s guidance General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products
Internal collaboration with OPQ’s OTR.
In vivo predictive method for determining opioid availability following chewing of solid oral opioids
OGD collaborated with OPQ’s OTR to develop an in-vitro chewing method that in combination with a PBPK-based IVIVC model can predict in vivo opioid availability following chewing. The in vitro chewing method was optimized based on available in-house PK data for chewed and ingested tablets. In addition, a PBPK model was developed to establish a mechanistic IVIVC for intact, chewed and crushed forms of Hysingla, using an in vivo predictive dissolution method. Erweka DRT 3 apparatus with a gap size of 4.3 mm is useful in simulating the in vivo chewing action and the IVIVC model for hydrocodone bitartrate ER tablet can be used to predict in vivo PK. FDA also successfully developed a Level A IVIVC link for in vitro dissolution data and in vivo PK after oral administration of intact, milled, and chewed hydrocodone bitartrate ER tablets with a correlation coefficient (R2) above 0.9.
PBPK/CFD predictions of deterrence of nasal insufflation
OGD collaborated with OPQ’s OTR to investigate in vitro dissolution and diffusion (VDC apparatus and USP IV flow-through cell dissolution apparatus); in vitro nasal deposition using an anatomically realistic nasal cavity model; and CFD analysis of regional deposition of milled particles in a respiratory model. The in vitro data obtained from the studies will be used to inform and verify in silico modeling that will be designed to provide relationships between particle size of physically altered drug product and systemic absorption of OxyContin. PBPK modeling using the in vitro data obtained will be developed to predict the in vivo PK of milled OxyContin tablets insufflated by recreational opioid users in an insufflation PK study that will be conducted as part of a GDUFA funded study. That study will inform future guidance development for AD generic opioid drug products.
Quantitative analysis of PK/PD relationship of AD opioid products
A critical path grant was awarded to Zhichuan (Matt) Li from OGD’s Office of Research and Standards for collaboration with the Office of Clinical Pharmacology in the Office of Translational Sciences. The study is ongoing.
Key Outcomes
The collaborative research funded under GDUFA supported FDA’s development of its guidance General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products. This guidance provides a general framework and approach for designing in vitro and in vivo studies to compare the abuse deterrence of generic ADF opioid products with their respective brand products. Specifically, the guidance shares the Agency’s current thinking on the general principles for evaluating abuse deterrence of generic solid oral opioid drug products that are intended to be duplicates of opioids formulated to incorporate physical or chemical barrier, agonist/antagonist, aversive agent, and combination of two or more of these technologies for all potential routes of abuse (i.e., oral, parenteral, nasal, and inhalation routes). FDA will also publish product-specific guidances (PSGs) for ADF opioid products to align with the general guidance.
FDA held public meetings with healthcare providers, pharmaceutical industry, academics, researchers, patient advocates, government entities, and other interested persons to discuss scientific issues related to ADF development and evaluation. FDA will continue to assess the state of science and, as novel technologies develop, will address them by issuing guidances, as appropriate.
Future Directions
Further research is needed to help standardize comparative abuse deterrence evaluation and use in vitro comparisons when appropriate. The ADF research goal in the next five years of GDUFA (2017-2022) is to continue and complete the abovementioned research projects and to develop and revise PSGs for ADF generic opioids. The long-term research plan will focus on advancing research to evaluate abuse deterrence in the nasal and oral routes.
With the advent of AD technologies and emerging ADF opioid products in the pharmaceutical market, regulatory efforts are underway to identify more efficient in vitro and in vivo assessment tools for comparing generic and brand ADF products. From the perspective of generic drug review, the recommended studies for an ANDA submission should meaningfully and reliably evaluate the comparative abuse deterrence between reference and generic ADF opioid products. Approval of generic ADF opioid products will ensure access to safe and effective analgesics for patients who need them while contributing to the fight against the nation-wide epidemic of opioid abuse.
Outcomes
General Guidance
Product-Specific Guidances
- Posting of Draft product-specific guidance on Hydrocodone Bitartrate extended-release tablet (October 2016)
- Posting of Draft product-specific guidance on Morphine Sulfate extended-release tablet (December 2016)
- Revision of Draft product-specific guidance on Oxycodone Hydrochloride extended-release tablet (July 2010; revised in October 2016)
- Posting of Draft product-specific guidance on Oxycodone extended-release capsule (December 2016)
Posters and Presentations
- Boyce H, Hoag SW. Excipient properties affecting the mechanical performance of abuse deterrent formulations. FDA Public Meeting (Development, Assessment and Regulation of Abuse-Deterrent Formulations of Opioid Medications), Oct 30, 2014, Silver Spring, MD.
- Boyce H, Ashour A, Deshpande T, Hoag SW. Quantification of polymer-excipient concentration in commercial harmaceutical drug products by diffuse reflectance near infrared spectroscopy. International Diffuse Reflectance Conference, Aug 2-8, 2014, Chambersburg, PA.
- Byrn S, Smith DT, Fang K, Hajec C. Failure modes of oxycodone and oxymorphone products. FDA Public Meeting (Development, Assessment and Regulation of Abuse-Deterrent Formulations of Opioid Medications), Oct 30, 2014, Silver Spring, MD.
- Smith DT. Research opportunities in evaluating abuse deterrent opioid formulations. FDA Public Meeting (Regulatory Science Initiative Part 15), May 16, 2014, Silver Spring, MD.
- Boyce H, Smith D, Gurvich V, Byrn S, Hoag SW. Can we standardize household tools? Category 1 Focus Group Meeting. Nov 4, 2015, Washington DC.
- Boyce H, Smith DT, Byrn S, Saluja B, Qu W, Gurvich VJ, Hoag SW. In vitro method to assess performance of abuse deterrent formulations for nasal route of abuse. American Association of Pharmaceutical Scientists (AAPS) Annual Meeting, Oct 25-29, 2015, Orlando, FL.
- Boyce H, Smith DT, Byrn S, Saluja B, Qu W, Gurvich VJ, Hoag SW. Investigation of abuse deterrent properties of sintered polyethylene oxide and hypromellose placebo tablets. AAPS Annual Meeting, Oct 25-29, 2015, Orlando, FL.
- Smith DT. Solid state chemistry and regulatory science of abuse deterrent medications. FDA Public Meeting (Regulatory Science Initiative Part 15), June 5, 2015, Silver Spring, MD.
- Boyce H, Hoag SW. Analysis of polyethylene oxide in sintered pharmaceutical tablets by transmission Raman spectroscopy. SCIX Conference, Sept 18-23, 2016, Minneapolis, MN.
- Zhao L. Foundations of PK comparisons of generic opioids to RLDs with labeling describing abuse-deterrent properties. FDA Public Meeting (Pre-market Evaluation of AD Properties of Opioid Drug Products), Oct 31, 2016, College Park, MD.
- Lionberger R. Introduction to FDA’s draft guidance on the General Principles for Evaluation of Abuse Deterrence of Generic Solid Oral Opioid Drug Products. FDA Public Meeting (Pre-market Evaluation of AD Properties of Opioid Drug Products), Oct 31, 2016, College Park, MD.
- Boyce H, Smith DT, Byrn S, Saluja B, Qu W, Gurvich VJ, Hoag SW. Influence of key polymer attributes, manufacturing conditions, and sintering on abuse deterrence of physical barrier type abuse deterrent formulations. AAPS Annual Meeting, Nov 13-17, 2016, Denver, CO.
- Boyce H, Smith DT, Byrn S, Saluja B, Qu W, Gurvich VJ, Hoag SW. Use of a vertical diffusion cell for the in vitro assessment of abuse deterrence of comminuted abuse deterrent formulations. AAPS Annual Meeting, Nov 13-17, 2016, Denver, CO.