ESG Chapter 2 Overview of The Registration Process
ESG User Guide - Table of Contents
Chapter 2 Overview of the Registration Process
Overview of the Registration Process
Registering to use the FDA ESG involves a sequence of steps that are to be conducted for all submitters and types of submissions. The first steps in the process are designed to ensure that the FDA ESG can successfully receive electronic submissions and that the electronic submissions are prepared according to published guidelines. The testing phase is done using the FDA ESG test system. Once the sender has passed the testing phase, an account will be set up allowing the submissions to be sent to the FDA ESG Production System.
The following diagram illustrates the steps in the process. The remaining sub-sections in Section 2 explain each of the steps in turn.
Figure 1: Overview of the Registration Process
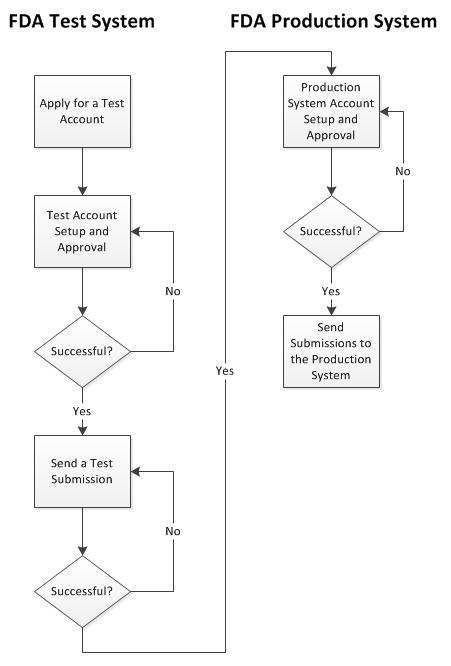
2.1 Apply for a Test Account
Organizations that wish to submit electronically to the FDA must apply for an account to establish themselves as Transaction Partners. The term "Transaction Partner" refers to:
An external entity authorized by the FDA to submit electronic submissions. Authorization includes agreement to regulatory conditions, successful completion of a certification process, and FDA administrative inclusion as a Transaction Partner.
Application for a test account must be initially requested for the FDA ESG. This is done to enable Transaction Partners to send a test submission to the FDA ESG.
Applying for an account involves information-sharing activities between the Transaction Partner and the FDA to set up transmission, receipt, and identification parameters. This ensures the correct identification of the Transaction Partner to the FDA. Digital certificate information is provided to the FDA as part of the application.
2.2 Test Account Setup and Approval
The account application is reviewed by the FDA ESG Administrator. The Administrator verifies that a letter of non-repudiation agreement is on file and that the digital certificate conforms to the X.509 version 3 standard and that all data fields in the Issuer and Subject fields are completed (see Appendix C., Digital Certificates for more information). The Administrator will also communicate with the Transaction Partner to confirm the application information. If these conditions are met, a test account is set up and connections to the FDA ESG test system are established before the submitting organization is approved as a Transaction Partner.
2.3 Send a Test Submission
By sending a test submission, the Transaction Partner ensures the following conditions are met:
- The test submission is received by the FDA ESG. A notification is sent by the FDA ESG confirming that the submission was successfully received.
- The submission is routed to the correct Center.
- The submission is prepared according to regulatory guidelines. The Center sends an acknowledgement confirming that the submission was prepared correctly.
2.4 Set Up a Production Account
Once you completed the test account setup and approval process, and the guidance compliant submission meets FDA requirements, your account will be migrated to production.
2.5 Send Submissions to the Production System
After completion of these steps, the Transaction Partner is enabled and approved to send submissions to the FDA ESG. The Production System Account allows the Transaction Partner to send any of the supported submission types to the FDA. However, the FDA will only process those submission types for which the Transaction Partner has received prior approval.
NOTE: It is the responsibility of the Transaction Partner to consult the appropriate FDA Center for information on formats, deadlines, and other information or procedures for submissions.
