Procedure for Requesting NOAA/FDA Analysis of Seafood from a State Harvesting Area Impacted by the Deepwater Horizon Oil Spill
Gulf of Mexico Oil Spill Main Page
Introduction
The National Oceanic and Atmospheric Administration (NOAA) in consultation with the U.S. Food and Drug Administration (FDA), the Environmental Protection Agency and state health and fisheries agencies in the Gulf region have established a protocol for use in re-opening oil-impacted areas closed to seafood harvesting. Once oil or chemical contaminants are visually observed on the surface (or subsurface based on shoreline staining, snare sentinel monitoring or water column testing), it is recommended that fisheries in the area be closed until the area is free of oil that can serve as a source of contamination to seafood and subsequent testing in accordance with the Protocol for Interpretation and Use of Sensory Testing and Analytical Chemistry Results for Re-Opening Oil-Impacted Areas Closed to Seafood Harvesting demonstrates the safety of seafood for human consumption and animal feed.
Consideration for re-opening an area within a state’s waters to seafood harvesting shall be initiated by the state agency or agencies responsible for the fishery and for the safety of the seafood for consumption. In the case of molluscan bivalve shellfish, that agency shall be the State Shellfish Control Authority (SSCA). The decision to request re-opening should be based on an assessment of the area with consideration given to the below factors at a minimum. If the state’s assessment indicates the re-opening of the area is appropriate, the state agency should provide in writing their determination to NOAA and FDA, which may only be one page (or longer if needed). States are encouraged to use the following email address to forward the written documentation and to ensure a timely response: nmfs.dwh.fda.dfs@noaa.gov.
NOAA and FDA shall review, and comment if needed, on the request to analyze re-opening samples. Any clarification required as part of the review process will be immediately directed to the agency point of contact provided in the initial request and should occur the same day the request is received. Once NOAA and FDA concur with the request to analyze samples for the purpose of re-opening a harvest area, the state agency or agencies will be notified immediately and asked to work directly with the National Seafood Inspection Laboratory (NSIL) on shipping instructions, shipping location and to address any additional questions related to sensory and chemical testing sample collection (contact information below).
When a state believes its waters are ready to be re-opened and plans to initiate the re-opening process, the requested written statement should outline the decision used to make the determination to re-open and consider the following factors to ensure proper prioritization for timely analysis:
Factos to Consider for Re-opening
- If closure was precautionary only: area may be re-opened without testing, however the agency may want to document relevant factors below for their record.
- Nature of observed oil that resulted in closure
- Tar balls
- Oil sheen
- Very light oil
- Light oil
- Moderate oil
- Heavy oil
- Spatial extent of observed oil
- Sub-surface oil and its spatial extent, if known
- Elapsed time since closure
- Elapsed time since last observance of oil (excluding possible oil sheen that may be present)
- Potential for future impact based on
- Presence of oil in adjacent or nearby areas
- Prevailing currents
- Prevailing winds
- Likelihood that fisheries will be impacted by oil movement within 7 - 10 days
- Nature of oil impact based on other available sources of information and monitoring
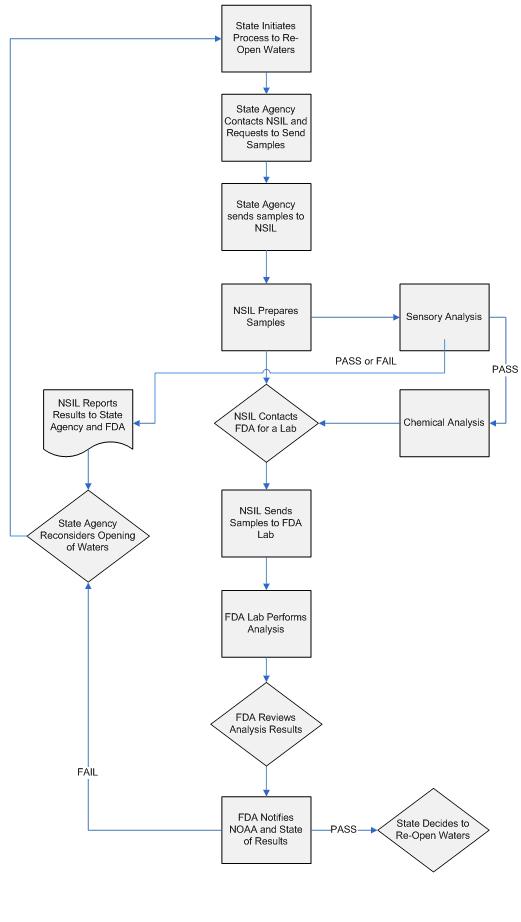
Once the area has been found ready to be re-opened the following flowchart is to be followed:
Sample Routing and Reporting
The urgency of the coordinated response to the Deepwater Horizon oil spill has required federal and state agencies to redefine their normal processes in order to expedite sample analyses and reporting. Therefore, state agencies are requested to utilize the following procedure to have their samples analyzed by NOAA/FDA to minimize communication difficulties and to keep a strong chain of custody procedure in place.
State Agency Decides to Re-Open – Once the oil spill has been contained and/or the oil has receded from state waters for a sufficient period of time, the applicable state agency will determine it is time to re-open a specific area for seafood harvesting. The state agency determines if the opening will be for all seafood harvested from a particular area or for specific species. The state agency should provide the information discussed on page 1 and forward the information via the email address provided. The state agency will then follow the agreed-upon protocol for sampling and collect samples from the area using a state-created sampling plan. The sampling plan should consider worst case conditions regarding the location and severity of the oil impacting the harvest area, hydrographic information and location and availability of harvestable recourses.
State Agency Sends Samples to NSIL – Before the samples have been collected the state agency contacts NSIL in Pascagoula, MS to confirm where to send the samples. NSIL will determine if samples are to be sent directly to them for evaluation or another analytical testing site designated by NSIL for sensory pre-screening based on resource availability.
National Seafood Inspection Laboratory
NOAA Fisheries
3209 Frederic Street
Pascagoula, MS 39567
Phone: 228-769-8964
Contact: Sample Custodian NMFS.DWH.FDA.DFS@noaa.gov
NSIL Prepares Samples for Analyses – Upon receipt, NSIL personnel prepare the samples for sensory evaluation and possible chemical analyses.
Sensory Analyses – Sensory analyses are performed on the samples per the criteria described in the agreed-upon re-opening protocol. The results are summarized and reported by the sensory panel. If the samples pass the protocol criteria for sensory analyses, chemical analyses will be performed on composite tissue samples per the agreed-upon protocol for re-opening. NSIL will communicate the results of the sensory analyses to the FDA and to the state contacts who submitted the re-opening samples.
NSIL Contacts FDA Labs – If the samples pass sensory analyses, NSIL will contact FDA through the designated sample email box. NSIL will provide FDA with both the sensory results and a copy of all the sampling labels to be submitted for chemical analysis. FDA will then identify a laboratory for chemical analysis and inform both NSIL and the chemistry laboratory of the impending shipment of samples. NSIL will send the samples to the laboratory identified for analyses.
Chemical Analyses – Upon receipt of the re-opening samples from NSIL, the lab will cross-reference the labeling on the samples with the database and information received from FDA. The lab will then perform the chemical analyses and provide the results to FDA for an internal review.
Reporting of Results – Following successful FDA review, the results are reported to NSIL and the state contacts who originally submitted the samples through the DFSR email box. After samples pass sensory analyses, FDA will be the primary point of contact for all issues and communication regarding the results and progress of the re-opening samples.
* Field sample collection - Samples for sensory and chemical analyses should be collected at the same time. See the Total Field Collection column in the table on this page:
Sensory Analyses – Up to 6 sub-samples per seafood type (3 sub-samples for oysters) from each sample location in the area under consideration for re-opening must be tested. A sub-sample will consist of an individual organism for legal size finfish and multiple organisms for shrimp, crab and oysters, depending on the intact animal type.
Chemical Analyses – For crabs specifically, a sample of edible muscle tissue from a minimum of ten (10) individuals, of legal size if available, should be collected from each sampling location. Tissue samples from individual crabs will be combined to make separate composite samples of the muscle tissue (of at least 200 grams). For all other seafood, a sample of edible tissue from a composite (of at least 200 grams) from a minimum of 15 oysters, 0.5 lb of shrimp and 6 finfish (only like family or species of finfish will be composited, non-similar types of finfish will be analyzed separately) collected at or near each sample location is requested. All samples should be collected from sites selected as commonly used fishing grounds or normally harvestable molluscan shellfish beds. Finfish samples may be collected over a period of consecutive days in order to obtain the minimum amount needed for analyses. (The number of total consecutive days should be limited as much as possible to reflect specific conditions during a specific time period.)
Field collection steps:
- Collect samples directly from the harvest area to be considered for re-opening consistent with existing individual state guidelines for commercial harvest (obtain all available sample location information, i.e. precise description, latitude/longitude or similar GIS coordinates., etc.).
- Rinse sample at harvest location in surrounding water, remove loose dirt and debris.
- Wrap finfish and oysters individually, and shrimp as a whole sample, in double layers of heavy-duty aluminum foil (dull side touching sample) in the field if possible. Crabs may be wrapped at the time of shipping. Limit field exposure to petroleum products during harvest, including engine exhaust from harvest vessel.
- Ensure that the sample is not touched by plastic to limit any potential petroleum odor transfer.
- Immediately chill the samples, 4 ºC (39 ºF) or below.
- Maintain in insulated, clean, shipping containers (odor-free if possible to limit any additional odor influence on the sample, i.e. disposable Styrofoam cooler may be preferred to new cooler if “new cooler smell” is present).
- Chill - If ice is used, prevent samples from coming in contact with melted water. If frozen gel packs are used, separate them from product with a layer of heavy-duty aluminum foil.
Seafood Samples to be Collected at each Sample Location
| Number of Animals per Subsample |
Sensory Testing | Chemical Testing2 | Total Field Collection1 (Animals per Sample Location) |
|
|---|---|---|---|---|
| # of Subsamples per Sample Location | Field Collection1 (Animals per Sample Location) |
Individual Animals Needed per Sample Location |
||
| Crabs: 6 (~2 lbs) | 6 | Collect 36 | 10 | Collect 46 |
| Oysters: 10 | 3 | Collect 30 | 15 | Collect 45 |
| Shrimp: 0.5 lbs | 6 | Collect 3 lbs | 0.5 lbs | Collect 3.5 lbs |
| Fin Fish: 1 fish | 63 | Collect 6 fish | 63 | Collect 6 - 12 |
| Sufficient material must be provided to be able to perform the necessary sensory analyses. Providing the amounts per sample indicated above will meet this need. |
||||
1Field collections methods should be similar to commercial harvest methods.
2Animals from a sampling station will be combined into a composite sample for each station.
3Fish should be large enough or in sufficient quantity to provide at least 0.5 lb sample size for each sensory evaluation and chemistry testing. For large fish, fewer fish may be needed for both sensory and chemistry testing with one filet going to sensory and one filet to chemistry. (E.g. fish over 10 lbs, a sample unit is 6 lbs of filet with skin on.) For small fish lacking filet size of at least 200 g individually, collect (6) 0.5 lb sample units for sensory and (6) 0.5 lb sample units for chemistry. (E.g. for butterfish and menhaden collect (6) 0.5 lb sample units for sensory and (6) 0.5 lb sample units for chemistry.)
All labeling should be on the plastic over-wrap (i.e. whirl-pac, zip-loc, etc) used around the foil-wrapped samples listed above – securely affix the label to the sample or place the label information in a second whirl-pac or zip-loc bag and place that sealed bag inside the first bag with the sample.
- All samples should be individually identified with the following included in the title “DEEPWATER HORIZON RE-OPENING SAMPLE.”
- Information on the label must include the Unique Sample ID Number to the State and the species
- Include state point of contact: Name, Telephone Number and Email Address
- Provide the following sample collection information with each sample:
- State and Agency
- Unique Sample ID Number to the State
- Date Collected
- Harvest Location (include Lat/Long)
- Species
- Number of Animals
- Name of Collector
Storage/shipping preparation steps:
- Place in freezer at -20 ºC (-4 ºF) or lower for approximately 30 minutes to immobilize the sample (i.e. for crabs).
- Animals should be wrapped individually in double layers of heavy-duty aluminum foil (dull side touching sample) as described previously.
- If sensory/chemical analysis will occur within 24 hours, ship to NSIL at 4°C, otherwise ship frozen at -20°C. Return to freezer until product is frozen if product is to be shipped by common carrier and not analyzed immediately.
Storage/shipping steps:
- Double bag the sample (i.e. whirl-pac, zip-loc, etc); expel almost all air from the bag (e.g.: push sides of bag completely then hold before closing).
- Other shipping containers are acceptable such as metal paint-style cans with securable lids.
- Ensure that the sample is not touched by plastic.
Shipping:
- Contact the National Seafood Inspection Laboratory in Pascagoula, MS for daily shipping instructions as samples may be diverted to an alternate screening site if a large amount of samples is anticipated.
- Chill samples as described above prior to shipping and ensure the samples remain at suggested temperature.
- Samples may be driven for same day delivery to the National Seafood Inspection Laboratory, Pascagoula, MS.
- Samples may be shipped frozen by common carrier for next day delivery.
Questions can be directed to the Interagency Liaison Officers:
Steven Wilson
Seafood Inspection Program
National Marine Fisheries Service
1315 East West Highway
Silver Spring, MD 20910
301-713-2355
Steven.Wilson@noaa.gov
Peter Koufopoulos
Office of Food Safety
Center for Food Safety and Applied Nutrition
U.S. Food and Drug Administration
College Park, MD 20740
301-436-1420
Peter.Koufopoulos@fda.hhs.gov