How GMOs Are Regulated in the United States
How GMOs are Regulated in the United States
Three federal agencies within the U.S. government work together to regulate most GMOs. “GMO” (genetically modified organism) has become the common term consumers and popular media use to describe a plant, animal, or microorganism that has had its genetic material (DNA) altered through a process called genetic engineering.
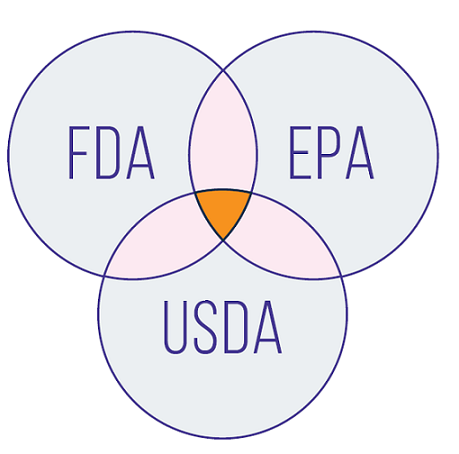
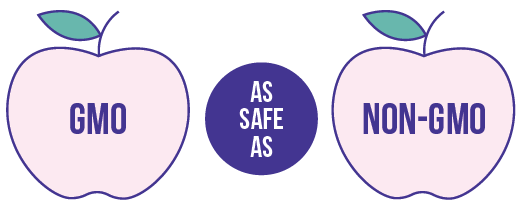
The U.S. Food and Drug Administration (FDA), U.S. Environmental Protection Agency (EPA), and U.S. Department of Agriculture (USDA) ensure that GMOs are safe for human, plant, and animal health.
These agencies also monitor the impact of GMOs on the environment.
The Coordinated Framework for the Regulation of Biotechnology, established in 1986, describes how the agencies work together to regulate GMOs.
U.S. Food and Drug Administration
FDA regulates most human and animal food, including GMO foods. In doing so, FDA makes sure that foods that are GMOs or have GMO ingredients meet the same strict safety standards as all other foods. FDA sets and enforces food safety standards that those who produce, process, store, ship, or sell food must follow, no matter how the foods are created.
U.S. Environmental Protection Agency
EPA is responsible for protecting human health and the environment, which includes regulating pesticides. EPA regulates the safety of the substances that protect GMO plants, referred to as plant-incorporated protectants (PIPs), that are in some GMO plants to make them resistant to insects and disease. EPA also monitors all other types of pesticides that are used on crops, including on GMO and non-GMO crops.
U.S. Department of Agriculture
The USDA Animal and Plant Health Inspection Service (APHIS) protects agriculture in the United States against pests and disease. APHIS sets regulations to make sure GMO plants are not harmful to other plants, and USDA’s Biotechnology Regulatory Services implements these regulations.
GMOs: Government Regulation and Your Safety
Hear from experts at FDA, EPA, and USDA as they discuss how federal agencies work together to ensure that GMOs are safe for human, plant, and animal health.
A Question of GMOs
Who makes sure GMOs are safe?
Many federal agencies play an important role in ensuring the safety of GMOs. As described in the Coordinated Framework for the Regulation of Biotechnology, multiple federal agencies work to ensure the safety of GMOs. Collaboration and coordination among these agencies help make sure food developers understand the importance of a safe food supply and the rules they need to follow when developing new products using genetic engineering.
FDA’s voluntary Plant Biotechnology Consultation Program evaluates the safety of food from new GMOs before they enter the market. This program allows developers to work with FDA on a product-by-product basis.
GMOs and Food Safety: A Guide for Health Educators
How does the Plant Biotechnology Consultation Program work?
The Plant Biotechnology Consultation Program is a voluntary program with four key steps:
- GMO plant developer meets with FDA about a potential new product for use in human and animal food.
- GMO developer submits food safety assessment data and information to FDA.
- FDA evaluates the data and information and resolves any issues with the developer.
- Consultation is complete once FDA has no more questions about the safety of the human and animal food made from the new GMO plant variety. Completed consultations are all made public.
The Program allows FDA to work with crop developers to help create a safe food supply. It also allows FDA to collect information about new foods. See a full list of GMOs that have gone through the Plant Biotechnology Consultation Program.
How can I tell if I’m eating GMOs?
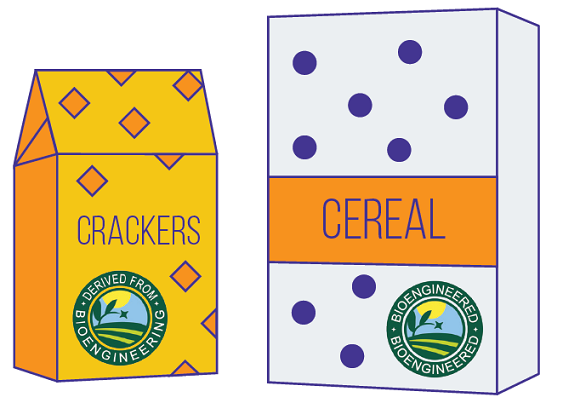
Certain types of GMOs have a disclosure that lets you know if the food, or ingredients you are eating, is a bioengineered food. The National Bioengineered Food Disclosure Standard defines bioengineered foods as those that contain detectable genetic material that has been modified through certain lab techniques and cannot be created through conventional breeding or found in nature.
The Standard establishes requirements for labeling foods that humans eat that are or may be bioengineered and defines bioengineered foods as those that contain detectable genetic material that has been modified through certain lab techniques and cannot be created through conventional breeding or found in nature.
The Standard requires that by 2022, food makers, importers, and certain retailers label foods that are bioengineered or have bioengineered ingredients. At that time, foods sold in the United States that meet the definition of bioengineered food must have information on their packaging using one of the approved methods, including text on the package that says “bioengineered food,” the bioengineered food symbol, or directions for using your phone to find the disclosure.
For more details on the labeling requirement for foods that are genetically modified or bioengineered, including sample labels, visit www.ams.usda.gov/be.
Is It Called GMO or Something Else?
Science and History of GMOs and Other Food Modification Processes
GMO Crops, Animal Food, and Beyond
How GMO Crops Impact Our World