Patient Medication Information (PMI)
Background
FDA is proposing to amend its human prescription drug labeling regulations to require a new type of Medication Guide—"Patient Medication Information” (PMI)—for prescription drug products used, dispensed, or administered on an outpatient basis, including blood and blood components transfused in an outpatient setting.
PMI will:
- Improve public health by providing patients with clear, concise, accessible, and useful patient information.
- Ensure that essential information for patients to use their prescription drug products safely and effectively is provided in a patient friendly manner.
- Consist of a single page document with standard content and formatting to make it easier for patients to access important information on drug products.
- Expand patient choice to include an electronic option to receive patient medication information in addition to the physical paper format.
FDA recognizes the importance of providing written information to patients about their prescription drug products. Evidence suggests that such information can help patients use their prescription drug products safely and effectively, which may reduce preventable adverse drug reactions and improve health outcomes. The layout and format of PMI is based on the prototypes that were developed through public meetings with interested parties through an iterative process. Prototypes were tested against each other and current medication guides to determine patient comprehension and retention.
PMI will highlight essential information that patients need to know about the prescription drug product, including basic directions on how to use the product. PMI will be an FDA-approved, one-page document that follows standardized format and content requirements. FDA-approved PMI will be given to patients with their prescription drug products to help them use their prescription drug products safely and effectively.
- PMI will be approved by the FDA.
- PMI will highlight the essential information that patients need to know about the prescription drug product, including basic directions on how to use the product.
- Authorized dispensers, such as pharmacists, would provide the FDA-approved PMI to patients with prescription drug products used, dispensed, or administered on an outpatient basis.
- Patients will have a choice to receive PMI in paper or electronic format. Paper is the default method of distribution and must always be provided unless a patient requests electronic delivery.
- The proposed rule, if finalized, will require applicants of all new and approved new drug applications (NDAs) and biologics license applications (BLAs) to create PMI for prescription drug products that are to be used, dispensed, or administered on an outpatient basis.
- The proposed rule will also require applicants of new and approved abbreviated new drug applications (ANDAs) that refer to a reference listed drug for which FDA has approved PMI to have PMI that is the same as that of the reference listed drug, except for certain differences in labeling permitted under the law. There will be an FDA template process for ANDAs without a reference listed drug.
- During the proposed 5-year implementation schedule of the final rule, the current regulations governing Medication Guides will remain in place but will no longer be applicable to a prescription drug product once that prescription drug product has FDA-approved PMI.
- PMI will replace current Medication Guides and Patient Package Inserts. PMI is not intended to replace the Prescribing Information, Instructions For Use, or patient counseling.
- Authorized dispensers, such as pharmacists, would provide the FDA-approved PMI to patients with prescription drug products used, dispensed, or administered on an outpatient basis.
- Patients will have a choice to receive PMI in either paper or electronic format. Paper is the default method of distribution and must always be provided unless a patient requests electronic delivery.
- PMI will be stored electronically in FDA’s labeling repository at https://labels.fda.gov.
- Prescription drug products that are given to patients in an inpatient setting, such as a hospital or nursing home, would not be required to have PMI. However, distribution of PMI would be required for prescription drug products a patient would continue to use at home, for example, as an outpatient.
- PMI will replace current Medication Guides and Patient Package Inserts. PMI is not intended to replace the Prescribing Information, Instructions For Use, or patient counseling.
PMI Development Past Events
- Jul. 1, 2014: Exploring the Promise of Patient Medication Information
- Feb. 23, 2011: Expert Workshop, Designing Pilot Programs to Evaluate Patient Medication Information
- Oct. 12, 2010: Public Workshop, Ensuring Access to Effective Patient Medication Information
- Sep. 27-28, 2010: FDA Part 15 Hearing, Developing and Distribution of Patient Medication Information for Prescription Drugs
- Jul. 21, 2010: Public Workshop, The Science of Communicating Medication Information to Consumers
- Sep. 24-25, 2009: Public Workshop, Providing Effective Information to Consumers about Prescription Drug Risks and Benefits
Comments on the Proposed Rule
FDA requested public comment on the entirety of the proposed rule, and specifically for certain elements, including, but not limited to:
- Whether the proposed format and content requirements support the accessibility of PMI for all intended users, including patients with limited health literacy.
- Actions applicants and other organizations might take to make PMI accessible to individuals with limited English proficiency.
- PMI requirements for which waivers could be requested and the criteria that FDA might consider when evaluating such requests.
The comment period on the proposed rule closed on November 27, 2023. FDA is in the process of reviewing the comments for consideration in issuing a final rule.
Contact Information
For questions related to the proposed rule:
Toll Free
(855) 543-3784, or
(301) 796-3400
Email: druginfo@fda.hhs.gov, or CDEROMP@fda.hhs.gov
Related Information
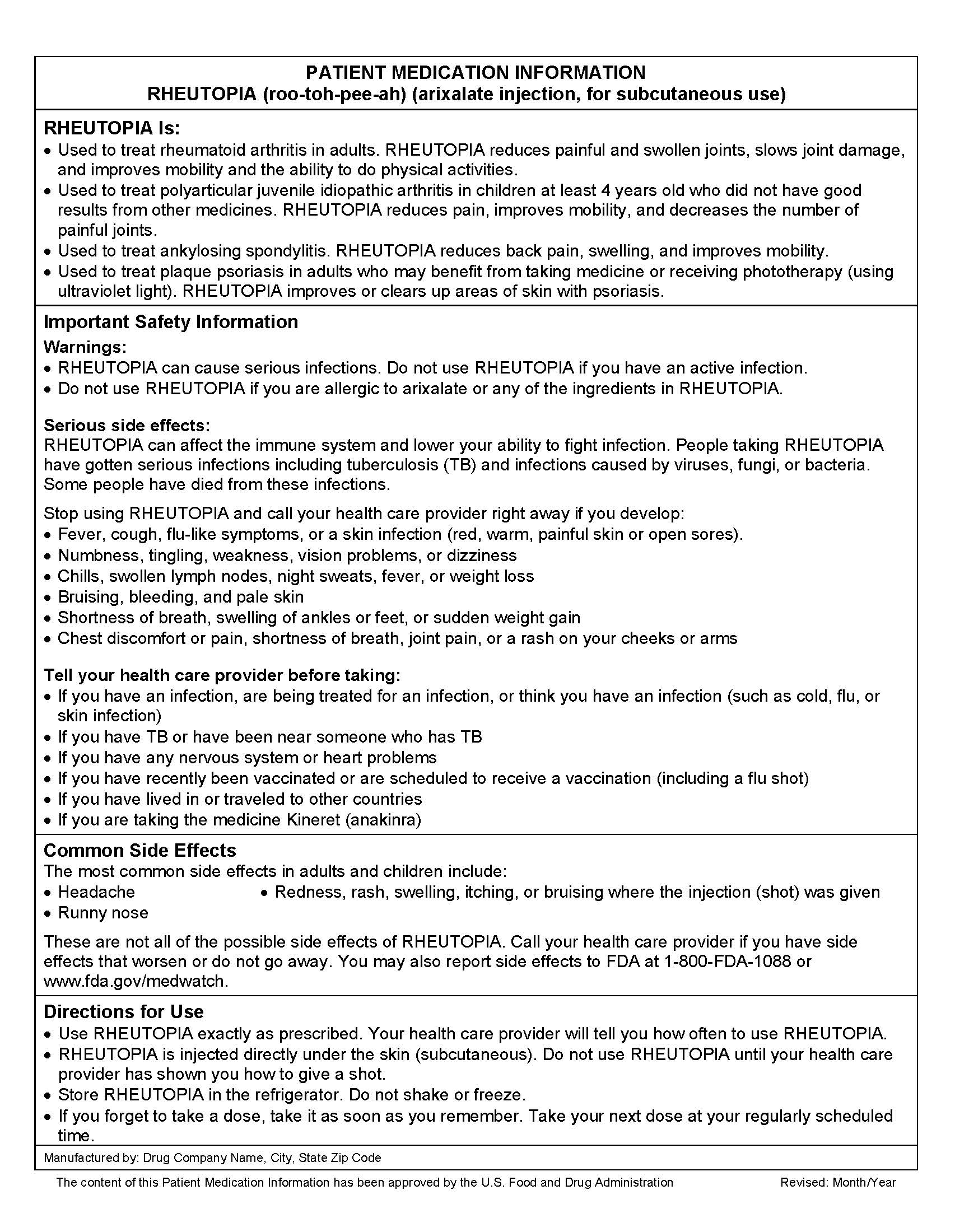
- Example of Patient Medication Information for a fictitious drug, Rheutopia
- Federal Register Notice
- Medication Guides
- Learn About Your Medicines
PMI Example for a Hypothetical Drug Product
The example below for a hypothetical product was created during the development of the proposed rule and may not represent requirements of a final rule, if issued.