Proposed Rule on Revising the National Drug Code Format
On July 22, 2022, FDA announced the availability of a proposed rule, Revising the National Drug Code Format and Drug Label Barcode Requirements (Docket No. FDA-2021-N-1351), that is intended to minimize the impact of FDA running out of ten-digit national drug codes (NDCs) by adopting a single, uniform 12-digit format for FDA-assigned NDCs. The NDC is the FDA standard for uniquely identifying drugs marketed in the United States. FDA is proposing to change the NDC to 12 digits in length with three distinct and consistent segments and one uniform format. Additionally, FDA is proposing to revise the drug product barcode label requirements.
NDCs are currently used across the healthcare system. Changes to the NDC would impact human and animal drug manufacturers, insurers/payors, wholesale distributors, drug databanks, pharmacies, hospitals, small clinics and healthcare practitioners, dentist offices, prisons, nursing care facilities, importers, federal agencies using the NDC, state and local governments, and other supply chain stakeholders that use FDA-assigned NDCs.
Because the Agency is running out of 10-digit NDCs, this rule, if finalized, would provide certainty and predictability to stakeholders by selecting a prespecified date for an NDC change. The COVID-19 pandemic significantly increased the rate at which NDC codes were issued.
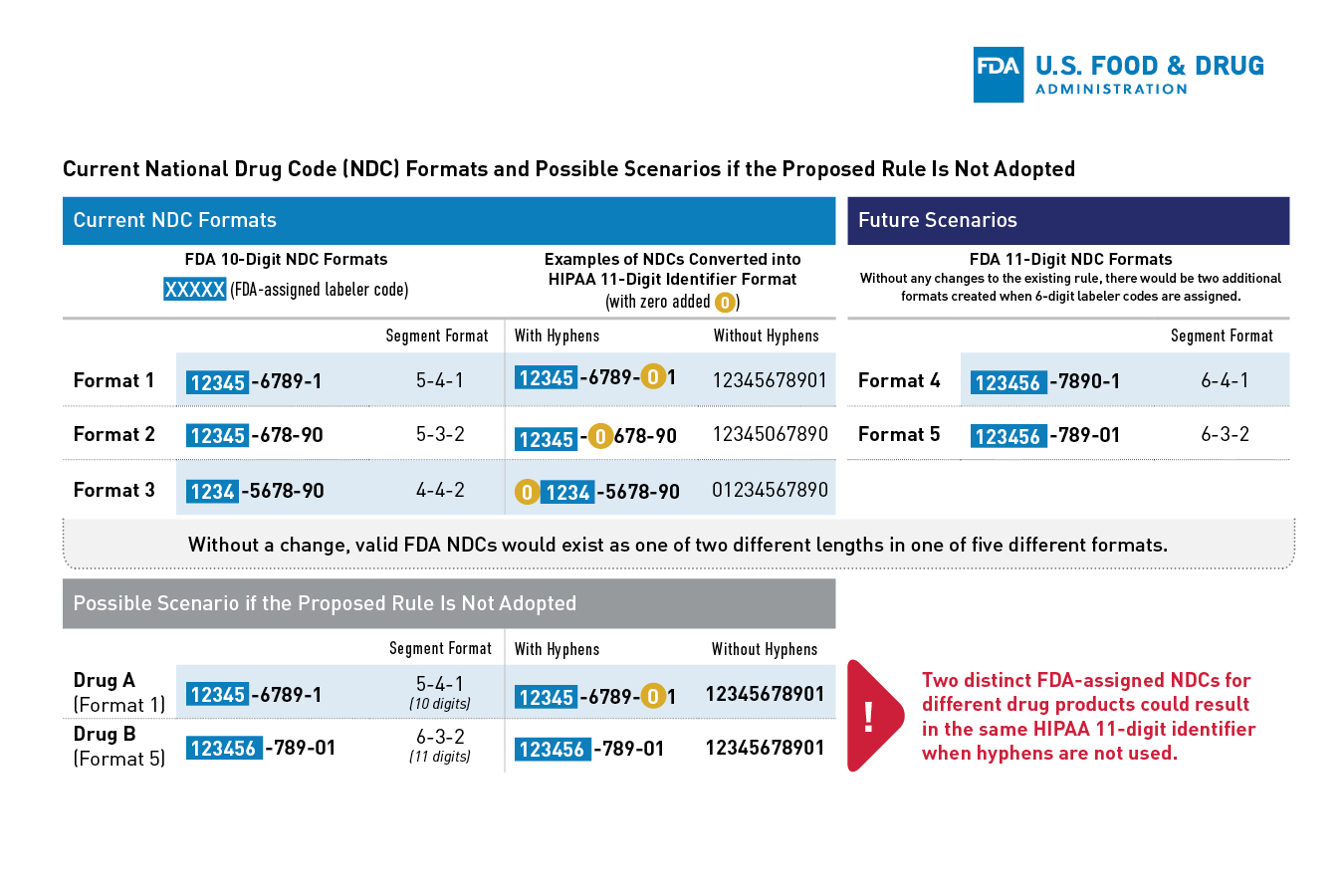
In light of the nature of the drug supply chain, FDA recognizes that it would be difficult for firms to immediately transition from a 10-digit NDC to a 12-digit NDC without a transition period and FDA is proposing a delayed effective date of the final rule. FDA anticipates that the Health Insurance Portability and Accountability Act of 1996 (HIPAA) standards and other code sets that currently require 10-digit native NDCs to be converted to 11-digit NDCs, will likely need to be updated in some manner.
One expected benefit of the proposed rule, if finalized, is that the proposed standardized format would facilitate the adoption of a single NDC format by all stakeholders. Such an adoption would eliminate the need to convert NDCs from one of FDA’s prescribed formats to a different standardized format used by other sectors of the healthcare industry (e.g., healthcare providers and payors).
Proposed Provisions
FDA is proposing to amend our regulations on foreign and domestic establishment registration and listing for drugs, including biological products and animal drugs. This proposed rule will affect drug products that are required to be listed under section 510 of the FD&C Act and 21 CFR part 207. Once effective, existing 10-digit NDCs will be required to convert to the new uniform 12-digit NDC format, and new NDCs will be assigned in the 12-digit format.
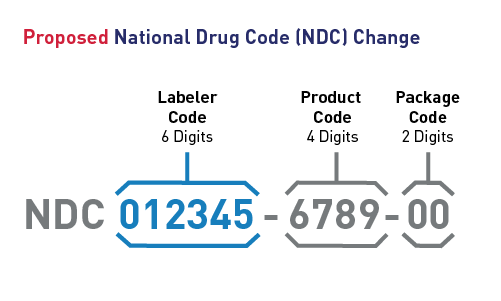
- Format of NDC: Under the proposed rule, the NDC would remain a three-segment numerical code consisting of the labeler code, the product code, and the package code. Specifically, we are proposing that the labeler code would be six digits in length, the product code would be four digits in length, and the package code would be two digits in length, a universal 6-4-2 format.
- Proposed Approach
- Final Rule Publishes: FDA is proposing an effective date five years after the publication of the final rule to allow stakeholders time to develop and implement changes to their systems.
- Effective Date: On the effective date identified in the final rule, FDA would begin assigning new NDCs in the uniform 12-digit format. Certain stakeholders that use FDA-assigned NDCs would need to have systems capable of handling the new, uniform, 12-digit NDC on the effective date of the final rule. Drug listing files submitted on or after this date would be required to use the 12-digit NDC. To reduce the burden on registrants, FDA does not intend to require resubmission of all existing drug listing files. Instead, FDA intends to convert existing NDCs on its own on the effective date by adding leading zeros to the appropriate segments.
- Transition period: A transition period for labeling will last three years after the effective date. FDA encourages manufacturers and distributors to include 12-digit NDCs on their drug labeling as soon as possible after the effective date. However, to aid with the transition, for a period of three years following the effective date, FDA does not intend to object to the continued use of 10-digit NDCs on the labeling of products that were assigned a 10-digit NDC prior to the effective date. FDA intends to mitigate the risk of medication error and confusion during the transition period by maintaining and publishing both the 10-digit and 12-digit NDC formats for specific drugs. This will provide stakeholders with a resource to confirm the identity of the drug in the event of any confusion.
- Labeling: Product labeling that includes a product’s 10-digit NDC would need to be updated to convert the 10-digit NDC to the standard 12-digit format. FDA is also proposing to revise the drug barcode label requirements to allow the use of either linear or nonlinear barcodes, so long as the barcode meets the prescribed standards. FDA is considering whether to further revise 21 CFR 201.25(c) to accommodate potential advances in technologies and standards development by allowing the use of unspecified automatic identification and data capture (AIDC) formats other than linear or non-linear barcodes in the future without the need to revise the regulation again.
Development of Proposed Rule
To create the proposed rule, FDA considered various stakeholders’ input. In 2016, FDA published the final rule: Requirements for Foreign and Domestic Establishment Registration and Listing for Human Drugs, Including Drugs That Are Regulated Under a Biologics License Application, and Animal Drugs and Listing. In 2018, FDA held a public hearing about several proposed formatting options FDA could adopt. Comments were in favor of FDA’s adoption of a single standardized format that could be used by all stakeholders. The majority of the commenters were in favor of FDA establishing a certain date when stakeholders would be required to have systems capable of handling the new format.
More Information
- Federal Register Notice Revising the National Drug Code Format and Drug Label Barcode Requirements (Docket No. FDA-2021-N-1351)
- Revising the National Drug Code Format and Drug Label Barcode Requirements (Proposed Rule) Regulatory Impact Analysis, Federal Register 87 FR 44038, Docket FDA-2021-N-1351
- National Drug Code Directory
- National Drug Code Directory Searchable Database
- Electronic Animal Drug Product Listing Directory