Drug Trials Snapshots: WAYRILZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the WAYRILZ Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
WAYRILZ (rilzabrutinib)
(WAY-rilz)
Genzyme Corporation
Approval date: August 29, 2025
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
WAYRILZ is a kinase inhibitor approved for the treatment of adult patient with persistent or chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
How is this drug used?
WAYRILZ is a 400 mg oral tablet that is taken twice daily.
Who participated in the clinical trials?
The FDA approved WAYRILZ based on evidence from a single clinical trial named LUNA-3 (NCT04562766), which enrolled 202 patients with immune thrombocytopenia lasting at least three months. The trial was conducted at 142 sites in 26 countries in Asia/Pacific, Europe, North America, South America, and Australia. Sixteen percent of patients were treated in North America. The same trial was used to evaluate the safety and efficacy of the drug.
How were the trials designed?
The safety and efficacy of WAYRILZ was evaluated in LUNA-3. In this study, patients were randomized to receive either WAYRILZ 400 mg twice daily or placebo (an inactive pill that is identical to WAYRILZ). Patients were two times more likely to receive WAYRILZ than the placebo. Randomized patients were to receive 24 weeks of blinded treatment (neither doctor nor patient knew which treatment the patient was receiving). However, after 12 weeks, patients without improvement in their platelet count were permitted to stop blinded treatment and initiate treatment with WAYRILZ. After the 24-weeks of blinded treatment, all patients could continue or initiate treatment with WAYRILZ. The main goal of the trial was to see if WAYRILZ could safely improve and maintain platelet counts in patients previously treated for ITP during the 24-week blinded treatment period.
How were the trials designed?
To participate in LUNA-3, patients were required to have had ITP for at least three months and have a history of having improved with a standard-of-care ITP treatment (intravenous immunoglobulin [IVIg/anti-D] or corticosteroid) but without a sustained increase in platelet count. Patients were permitted to have had their spleen removed. Assignment to the treatment arms (WAYRILZ or placebo) were made so that the percentage of patients who had had a spleen removed and the percentage of patients with platelet count <15 x 109/L were about the same between the two arms. Concurrent treatment with other ITP medications (oral corticosteroid and/or a thrombopoietin receptor agonist) were allowed, but the dose had to be stable before starting study treatment and had to remain the same through treatment on study. When platelet count was less than 20 x 109/L or if there was concerning bleeding, rescue medication to increase platelet counts or prevent bleeding was allowed.
DEMOGRAPHICS SNAPSHOT
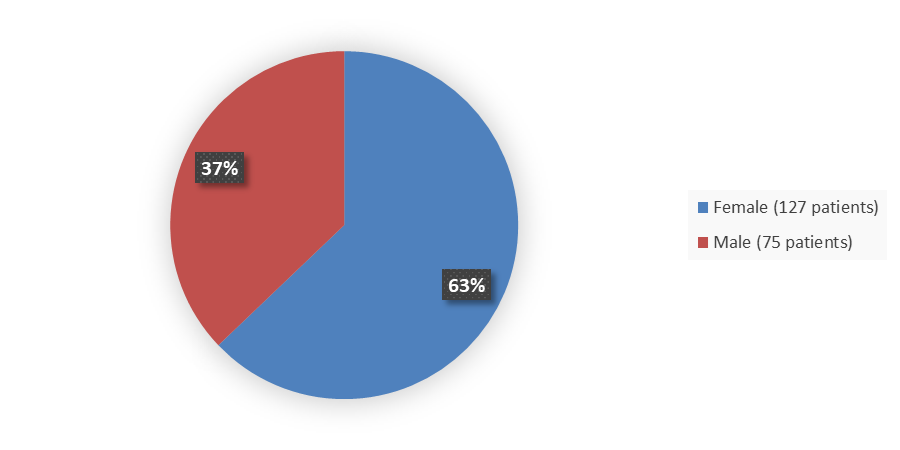
The primary efficacy population for this application included 202 patients with persistent or chronic ITP. Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the safety and efficacy of WAYRILZ.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
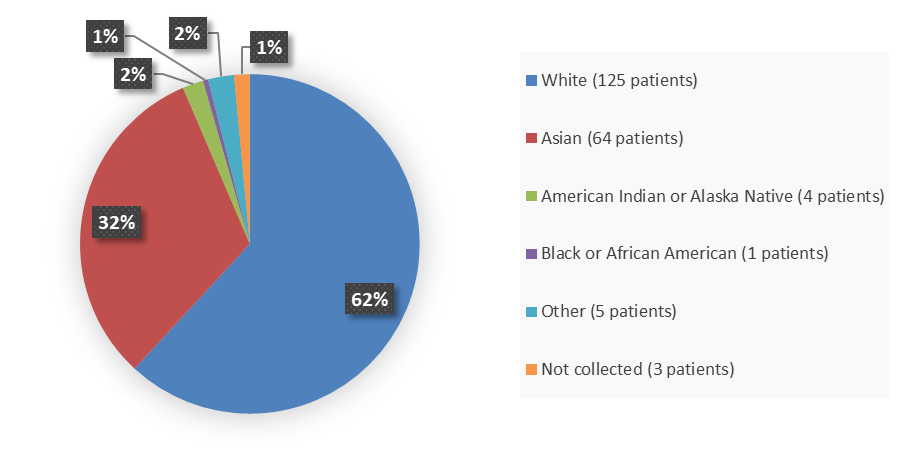
Figure 2 summarizes the percentage of patients by race who enrolled into the clinical trial used to evaluate the safety and efficacy of WAYRILZ.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
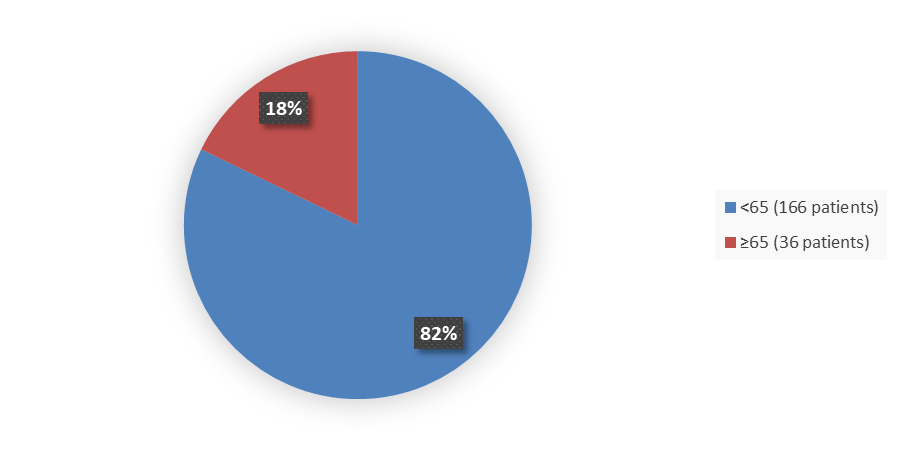
Figure 3 summarizes the percentage of patients by age enrolled into the clinical trial used to evaluate the safety and efficacy of WAYRILZ.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
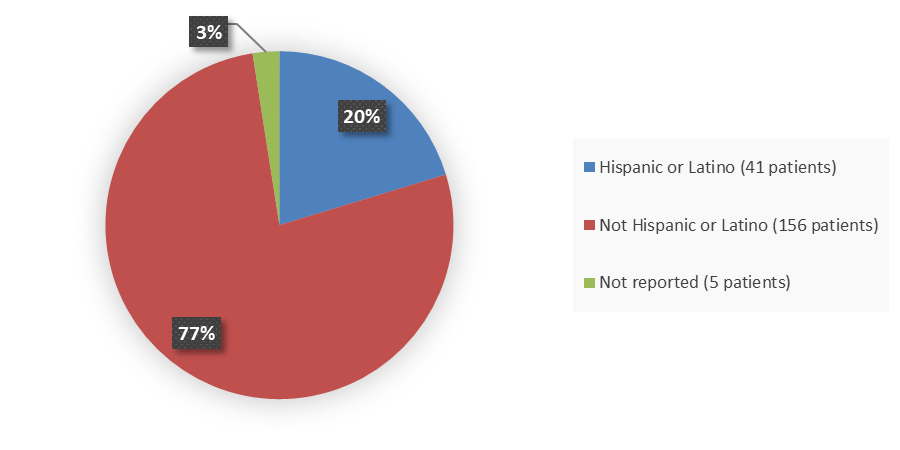
Figure 4 summarizes the percentage of patients by ethnicity in the efficacy population that enrolled into the clinical trial used to evaluate the safety and efficacy of WAYRILZ.
Figure 4. Baseline Demographics by Ethnicity
Source: Adapted from FDA Review
Who participated in the trials?
Table 1. Baseline Demographics
| Characteristic | WAYRILZ N=133 | Placebo N=69 | Total N=202 |
|---|---|---|---|
| Age, years | |||
| Median (min, max) | 46.8 (18, 80) | 46.0 (19, 79) | 47.0 (18, 80) |
| Sex, n (%) | |||
| Female | 78 (58.6) | 49 (71.0) | 127 (62.9) |
| Male | 55 (41.4) | 20 (29.0) | 75 (37.1) |
| Race, n (%) | |||
| White | 85 (63.9) | 40 (58.0) | 125 (61.9) |
| Black or African American | 1 (0.8) | 0 | 1 (0.5) |
| Asian | 40 (30.1) | 24 (34.8) | 64 (31.7) |
| Not reported | 1 (0.8) | 2 (2.9) | 3 (1.5) |
| Other | 3 (2.3) | 2 (2.9) | 5 (2.5) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 28 (21.1) | 13 (18.8) | 41 (20.3) |
| Not Hispanic or Latino | 103 (77.4) | 53 (76.8) | 156 (77.2) |
| Not reported | 2 (1.5) | 3 (4.3) | 5 (2.5) |
| Geographic region, n (%) | |||
| Asia/Pacific | 44 (33.1) | 26 (37.7) | 70 (34.7) |
| West Europe | 33 (24.8) | 16 (23.2) | 49 (24.3) |
| South America | 24 (18) | 11 (16) | 35 (17.3) |
| East Europe | 19 (14.3) | 13 (18.8) | 32 (15.8) |
| North America | 13 (9.8) | 3 (4.3) | 16 (7.9) |
Source: Adapted from FDA Review
What are the benefits of this drug?
In LUNA-3, a higher percentage (23%) of adults with persistent or chronic ITP taking WAYRILZ 400 mg twice daily achieved a durable platelet response compared to no patients taking the placebo treatment.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes efficacy results of WAYRILZ treatment in patients in LUNA-3.
Table 2. Efficacy Results, LUNA-3
| Endpoints | WAYRILZ N=133 | Placebo N=69 |
|---|---|---|
| Durable platelet response | ||
| n (%) | 31 (23.3) | 0 (0.0) |
| Risk difference (95% CI) vs. placebo | 23.1 (15.95, 30.31) | |
| p-valuea | <0.0001 | |
| Number of weeks with platelet response* | ||
| LS mean (SE) | 7.18 (0.747) | 0.72 (0.350) |
| LS mean difference (95% CI) vs. placebo | 6.46 (4.923, 7.990) | |
| p-valueb | <0.0001 | |
| Number of weeks with platelet response# | ||
| LS mean (SE) | 6.95 (0.749) | 0.64 (0.337) |
| LS mean difference (95% CI) vs. placebo | 6.31 (4.787, 7.831) | |
| p-valueb | <0.0001 | |
| Time (days) to first response (95% CI) | ||
| 1st quartile (25th percentile) | 10.0 (8.00, 15.00) | 65.0 (36.00, NA) |
| Median (50th percentile) | 36.0 (22.00, 44.00) | NA (NA, NA) |
| Hazard ratio (95% CI) vs. placeboc | 3.10 (1.948, 4.934) | |
| p-valued | <0.0001 | |
| Proportion of participants requiring rescue therapy | ||
| n (%) | 44 (33.1) | 40 (58.0) |
| Median time to first use of rescue therapy (days) | NA | 56 |
Source: Adapted from WAYRILZ Prescribing Information and FDA Review
* Number of weeks with platelet count ≥50,000/μL OR between ≥30,000/μL and <50,000/μL and at least doubled from baseline over the 24-week blinded treatment period in the absence of rescue therapy.
# Number of weeks with platelet counts ≥30,000/μL and at least doubled from baseline over the 24-week blinded treatment period in the absence of rescue therapy.
a Cochran-Mantel-Haenszel test adjusted by randomization stratification factors (splenectomy status (yes/no) and severity of thrombocytopenia (Inclusion Criteria 3 platelet counts <15,000/μL or ≥15,000/μL).
b Mixed-effect model with repeated measures on longitudinal binary data with treatment group as factor and adjusted by randomization stratification factor.
c Cox regression model adjusted by randomization stratification factor.
d Log-rank test adjusted by randomization stratification factor.
Abbreviation: CI, confidence interval; N, number of participants in study arm; n, number of participants with response; LS, least squares; SE, standard error; NA, not available
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: The observed effect of WAYRILZ was similar for males and females.
- Race: WAYRILZ worked similarly in Asian and White patients. The number of patients in other races was limited; therefore, differences in response for other races could not be determined.
- Age: The observed effect of WAYRILZ appeared similar in patients younger than 65 years and in patients aged 65 years and older.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Subgroup data from trials are presented for LUNA-3 in Table 3. Due to the small sample size, these exploratory analyses should be interpreted with caution.
Table 3. Subgroup Analyses for the Primary Endpoint of Durable Platelet Response by Subgroup, LUNA-3
| Subgroup | WAYRILZ N=133 n/Ns (%) | Placebo N=69 n/Ns (%) | Difference (95% CI) |
|---|---|---|---|
| Sex | |||
| Male | 8/55 (14.5) | 0/20 (0.0) | 14.5 (5.23, 23.86) |
| Female | 23/78 (29.5) | 0/49 (0.0) | 29.5 (19.37, 39.61) |
| Age group, years | |||
| <65 | 28/112 (25.0) | 0/54 (0.0) | 25.0 (16.98, 33.02) |
| ≥65 | 3/21 (14.3) | 0/15 (0.0) | 14.3 (-0.68, 29.25) |
| Race | |||
| White | 17/85 (20.0) | 0/40 (0.0) | 20.0 (11.50, 28.50) |
| Asian | 12/40 (30.0) | 0/24 (0.0) | 30.0 (15.80, 44.20) |
| Other | 2/8 (25.0) | 0/5 (0.0) | 25.0 (-5.01, 55.01) |
| Geographic region | |||
| Asia/Pacific | 12/44 (27.3) | 0/26 (0.0) | 27.3 (14.11, 40.43) |
| West Europe | 4/33 (12.1) | 0/16 (0.0) | 12.1 (0.99, 23.26) |
| East Europe | 8/19 (42.1) | 0/13 (0.0) | 42.1 (19.90, 64.31) |
| North America | 3/13 (23.1) | 0/3 (0.0) | 23.1 (0.17, 45.98) |
| South America | 4/24 (16.7) | 0/11 (0.0) | 16.7 (1.76, 31.58) |
Source: Adapted from WAYRILZ Prescribing Information and FDA Review
Abbreviations: CI, confidence interval; N, number of patients in treatment arm; n, number of patients meeting criteria; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
The safety of WALYRILZ was evaluated in 202 adults with persistent or chronic ITP who enrolled in LUNA-3. Among patients who received WAYRILZ for ITP, 50% received it for six months or longer, and 15% received it for over one year.
WAYRILZ may be associated with serious infections. Similar acting drugs (other bruton tyrosine kinase inhibitors) have been associated with liver toxicity, including drug-induced liver injury. In clinical trials of WAYRILZ in patients with ITP, elevations of liver tests (i.e., liver transaminases) occurred and were mild to moderate in severity. Studies in animals suggest WAYRILZ may cause fetal harm when given to women who become pregnant while taking the drug.
The most common adverse reactions (incidence ≥10%) were diarrhea, nausea, headache, abdominal pain, and COVID-19.
What are the possible side effects (results of trials used to assess safety)?
Table 4. Common Adverse Reactionsa in Patients With ITP During Double-Blind Period of LUNA-3
| Adverse Reaction | WAYRILZ, N=133 | Placebo, N=69 | ||
|---|---|---|---|---|
| All Grades % | Grade 3 or Higher % | All Grades % | Grade 3 or Higher % | |
| Diarrhea | 32 | 0 | 10 | 0 |
| Nausea | 20 | 0 | 6 | 0 |
| Headache | 18 | 0 | 7 | 0 |
| Abdominal pain | 18 | 0 | 1 | 0 |
| COVID-19 | 14 | 0.8 | 4 | 0 |
| Arthralgia | 9 | 0 | 4 | 0 |
| Dizziness | 8 | 0 | 1 | 0 |
| Nasopharyngitis | 7 | 0 | 3 | 0 |
| Vomiting | 7 | 0 | 1 | 0 |
| Dyspepsia | 5 | 0 | 0 | 0 |
| Cougha | 5 | 0 | 0 | 0 |
Source: Adapted from FDA Review
a Adverse reactions that occurred in at least 5% of WAYRILZ treated patients and at least 3% higher than placebo
Abbreviations: ITP, immune thrombocytopenia
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar between White and Asian. The number of patients in other races was limited; therefore, difference in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients below and above 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5. Side Effects by Subgroup, Safety Population, LUNA-3
| Subgroup | All Grades WAYRILZ N=133 n/Ns (%) | All Grades Placebo N=69 n/Ns (%) |
|---|---|---|
| Sex | ||
| Female | 65/78 (83.3) | 39/49 (79.6) |
| Male | 46/55 (83.6) | 13/20 (65.0) |
| Age group, years | ||
| <65 | 92/112 (82.1) | 39/54 (72.2) |
| ≥65 | 19/21 (90.5) | 13/15 (86.7) |
| Race | ||
| American Indian or Alaska Native | 3/3 (100) | 1/1 (100) |
| Asian | 33/40 (82.5) | 15/24 (62.5) |
| Black or African American | 1/1 (100) | 0/0 (NA) |
| White | 71/85 (83.5) | 34/40 (85.0) |
| Other | 2/3 (66.7) | 1/2 (50.0) |
| Not collected | 1/1 (100) | 1/2 (50.0) |
| Ethnicity | ||
| Hispanic or Latino | 26/28 (92.9) | 10/13 (76.9) |
| Not Hispanic or Latino | 83/103 (80.6) | 40/53 (75.5) |
| Not reported | 2/2 (100) | 2/3 (66.7) |
Source: Adapted from FDA Review
Abbreviations: N, number of patients in treatment arm; n, number of patients with adverse event; NA, not applicable; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.