Drug Trials Snapshots: TERLIVAZ
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the TERLIVAZ Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
TERLIVAZ (terlipressin)
(TUR-lih-vaz)
MALLINCKRODT PHARMACEUTICALS IRELAND LTD
Approval date: September 14, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
TERLIVAZ is a vasopressin receptor agonist that is indicated to improve kidney function in adults with hepatorenal syndrome (HRS) with rapid reduction in kidney function.
How is this drug used?
TERLIVAZ is an injection that is administered every six hours for up to 14 days.
Who participated in the clinical trials?
FDA approved TERLIVAZ based on evidence from a clinical trial (CONFIRM) of 199 patients with HRS with rapid reduction in kidney function. The trial was conducted at 60 sites in two countries: the United States (55 sites) and Canada (5 sites). The same trial was used to assess both efficacy and safety.
How were the trials designed?
The effectiveness of TERLIVAZ was assessed in a double-blind study. Participants with HRS with rapid reduction in kidney function were randomly assigned to receive TERLIVAZ (199 participants) or a placebo (101 participants). Participants received either 0.85 mg of TERLIVAZ or a placebo every six hours as an injection in the vein (intravenous) for a maximum of 14 days. The dose was adjusted based on changes in kidney function.
How were the trials designed?
The safety and efficacy of TERLIVAZ was assessed in a multicenter, double-blind, randomized, placebo-controlled study (CONFIRM, NCT02770716). Patients with cirrhosis (liver scarring), ascites (fluid build-up in the abdomen), and a diagnosis of HRS with rapid progression were eligible to participate. All patients received intravenous albumin (protein) therapy. Patients with a baseline serum creatinine level >7.0 mg/dL, shock (sudden drop in blood pressure), and/or uncontrolled bacterial infection were excluded from the study.
The primary efficacy endpoint was the incidence of verified HRS reversal, defined as the percentage of patients with two consecutive days of serum creatinine levels of 1.5 mg/dL or less, obtained at least two hours apart, by Day 14 or the participant’s final day in the study. To meet the primary endpoint, patients also had to be alive and without renal replacement therapy (treatment for kidney failure, such as dialysis) at least 10 days after achieving verified HRS reversal.
DEMOGRAPHICS SNAPSHOT:
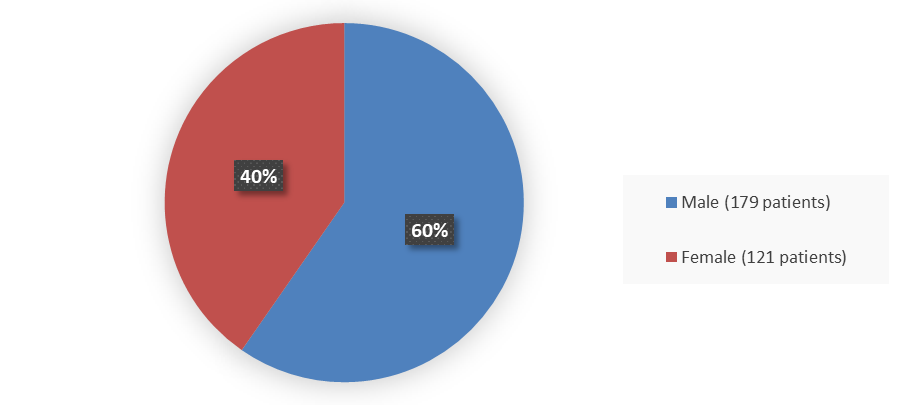
Figure 1 summarizes how many male and female patients were enrolled in the clinical trial used to evaluate the efficacy and safety of TERLIVAZ.
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
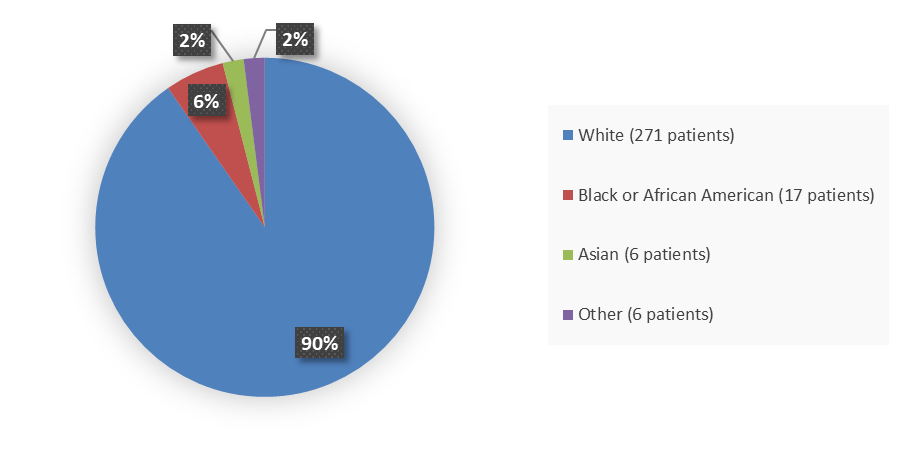
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy and safety of TERLIVAZ.
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
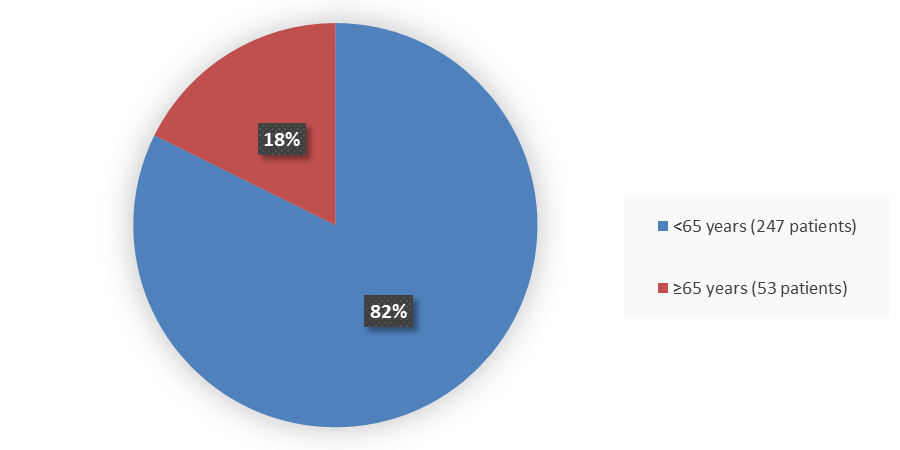
Figure 3 summarizes how many patients by age were in the trial used to evaluate the efficacy and safety of TERLIVAZ.
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
Figure 4. Baseline Demographics by Ethnicity (Efficacy Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes demographics of the patients in the clinical trial.
|
Characteristic |
TERLIVAZ N=199 |
Placebo N=101 |
|
Sex, n (%) |
|
|
|
Female |
79 (40) |
42 (42) |
|
Male |
120 (60) |
59 (58) |
|
Age, years |
|
|
|
Mean (SD) |
54.0 (11.3) |
53.6 (11.8) |
|
Median (min, max) |
55 (23, 78) |
55 (31, 82) |
|
Race, n (%) |
|
|
|
White |
177 (89) |
94 (93) |
|
Asian |
5 (2) |
1 (1) |
|
Black or African American |
12 (6) |
5 (5) |
|
Other or unknown |
5 (2) |
1 (1) |
|
Ethnicity, n (%) |
|
|
|
Hispanic |
32 (16) |
13 (13) |
|
Non-Hispanic |
165 (83) |
88 (87) |
|
Country of participation, n (%) |
|
|
|
United States |
178 (89) |
89 (88) |
|
Canada |
21 (11) |
12 (12) |
Source: FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
Kidney function improved in 29% of participants who received TERLIVAZ compared to 16% of participants who received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
A trial comparing TERLIVAZ to placebo in adults with hepatorenal syndrome evaluated the percentage of patients who had kidney function improvement (“verified HRS reversal”), defined as two consecutive days of serum creatinine levels of 1.5 mg/dL or less, obtained at least two hours apart, by Day 14 or the participant’s final day in the study. Improvement in kidney function was noted in 29% of participants in the TERLIVAZ group compared to 16% of participants in the placebo group.
Table 2 summarizes the efficacy results for the trial.
|
Parameter |
TERLIVAZ N=199 |
Placebo N=101 |
P-value |
|
Verified HRS reversal*, n (%) |
58 (29.1) |
16 (15.8) |
0.012 |
|
95% CI |
0.2, 0.4 |
0.1, 0.2 |
|
|
Durability of HRS reversala,b, n (%) |
63 (31.7) |
16 (15.8) |
0.003 |
|
95% CI |
03., 0.4 |
0.1, 0.2 |
|
|
Incidence of HRS reversala in the SIRS subgroup, n/Ns (%) |
28/84 (33.3) |
3/48 (6.3) |
<0.001 |
|
95% CI |
0.2, 0.4 |
0.0, 0.1 |
|
|
Incidence of verified HRS reversal without HRS recurrence by Day 30, n (%) |
48 (24.1) |
16 (15.8) |
0.092 |
|
95% CI |
0.2, 0.3 |
0.1, 0.2 |
Source: TERLIVAZ Prescribing Information
* Primary endpoint
a Patients with a SCr value of not more than 1.5 mg/dL while on treatment, by Day 14, or discharge.
b Patients with HRS reversal without renal replacement therapy to Day 30
Abbreviations: CI, confidence interval; HRS, hepatorenal syndrome; n, number of patients with HRS reversal; Ns, total number of patients for each specific subgroup and were assigned to that specific arm; SCr, serum creatinine; SIRS, systemic inflammatory response syndrome
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: TERLIVAZ worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how TERLIVAZ worked among races could not be determined.
- Age: TERLIVAZ worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3. Verified HRS Reversal Within Subgroups, ITT Population
|
Subgroup |
TERLIVAZ N=199 n/Ns (%) |
Placebo N=101 n/Ns (%) |
|
Overall |
58/199 (29) |
16/101 (16) |
|
Sex |
|
|
|
Female |
23/79 (29) |
7/42 (17) |
|
Male |
35/120 (29) |
9/59 (15) |
|
Race |
|
|
|
Asian |
1/5 (20) |
0/1 (0) |
|
Black / African American |
3/12 (25) |
2/5 (40) |
|
White |
53/177 (30) |
14/94 (15) |
|
Other |
1/5 (20) |
0/1 (0) |
|
Age, years |
|
|
|
<65 |
47/164 (29) |
14/83 (17) |
|
≥65 |
11/35 (31) |
2/18 (11) |
Source: Adapted from FDA review
Abbreviations: HRS, hepatorenal syndrome; ITT, intent-to-treat; n, number of patients with HRS reversal; Ns, total number of patients for each specific subgroup and were assigned to that specific arm
What are the possible side effects?
TERLIVAZ increases the risk of serious or fatal respiratory (breathing) failure. Patients with low oxygen in their blood should not start the medication. During treatment, patients should be monitored for breathing problems with a pulse oximeter, a tool that measures oxygen levels in the blood.
Side effects of TERLIVAZ may prevent patients from receiving a liver transplant. TERLIVAZ can cause ischemic events (that occur when blood does not reach certain parts of the body) that may require pausing or stopping treatment; the medication may also cause fetal harm when used during pregnancy.
The most common side effects include abdominal pain, nausea, diarrhea, respiratory failure, and dyspnea (difficulty breathing).
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes common adverse reactions that occurred in ≥4% of TERLIVAZ treated patients compared to placebo in the CONFIRM trial.
Table 4. Adverse Reactions Reported by ≥4 % of TERLIVAZ-Treated Patient
| Preferred Term |
TERLIVAZ n (%) |
Placebo n (%) |
|
Abdominal pain |
39 (19.5) |
6 (6.1) |
|
Nausea |
32 (16.0) |
10 (10.1) |
|
Respiratory failure |
31 (15.5) |
7 (7.1) |
|
Diarrhea |
26 (13.0) |
7 (7.1) |
|
Dyspnea |
25 (12.5) |
5 (5.1) |
|
Fluid overload |
17 (8.5) |
3 (3.0) |
|
Pleural effusion |
11 (5.5) |
0 (0.0) |
|
Sepsis |
11 (5.5) |
1 (1.0) |
|
Bradycardia |
10 (5.0) |
0 (0.0) |
|
Ischemia-related eventsa |
9 (4.5) |
0 (0.0) |
Source: TERLIVAZ Prescribing Information
a Ischemia-related events include: skin discoloration, cyanosis, ischemia and intestinal ischemia
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in side effects of TERLIVAZ among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5 summarizes the most common side effects (abdominal pain, nausea, and respiratory failure) by sex, race and age.
Table 5. Overview of Side Effects by Sex, Race, and Age in Trial MNK19013058, Safety Population
|
|
TERLIVAZ N=200 |
Placebo N=99 |
||||
|
Characteristic |
All Patients n (%) |
All Grades |
Grades 3 to 4 |
All Patients n (%) |
All Grades |
Grades 3 to 4 |
|
Sex, n (%) |
|
|
|
|
|
|
|
Female |
80 (40.0) |
77/80 (96.2) |
33/80 (41.2) |
41 (41.4) |
40/41 (97.6) |
11/41 (26.8) |
|
Male |
120 (60.0) |
111/120 (92.5) |
35/120 (29.2) |
58 (58.6) |
56/58 (96.6) |
13/58 (22.4) |
|
Race, n (%) |
|
|
|
|
|
|
|
White |
178 (89.0) |
167/178 (93.8) |
63/178 (35.4) |
92 (92.9) |
89/92 (96.7) |
21/92 (22.8) |
|
American Indian or Alaska Native |
2 (1.0) |
2/2 (100) |
0/2 (0) |
0 (0) |
0/0 (NA) |
0/0 (NA) |
|
Asian |
5 (2.5) |
5/5 (100) |
0/5 (0) |
1 (1.0) |
1/1 (100) |
1/1 (100) |
|
Black or African American |
12 (6.0) |
11/12 (91.7) |
5/12 (41.7) |
5 (5.1) |
5/5 (100) |
2/5 (40.0) |
|
Missing |
3 (1.5) |
3/3 (100) |
0/3 (0) |
1 (1.0) |
1/1 (100) |
0/1 (0) |
|
Age group, years, n (%) |
|
|
|
|
|
|
|
<65 years |
164 (82.0) |
153/164 (93.3) |
53/164 (32.3) |
82 (82.8) |
79/82 (96.3) |
21/82 (25.6) |
|
≥65 years |
36 (18.0) |
35/36 (97.2) |
15/36 (41.7) |
17 (17.2) |
17/17 (100) |
3/17 (17.6) |
Source: adae.xpt (eCTD seq 0022); FDA reviewer's analysis
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION