Drug Trials Snapshots: RELYVRIO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the RELYVRIO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
RELYVRIO (sodium phenylbutyrate and taurursodiol)
Reh-lee-vree-oh
Amylyx Pharmaceuticals Inc
Original Approval date: September 29, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
RELYVRIO is a drug that treats adult patients with amyotrophic lateral sclerosis (ALS).
ALS is a rare disease that attacks and kills the nerve cells that control voluntary muscles. Voluntary muscles produce movements such as chewing, walking, breathing, and talking. ALS causes the nerves to lose the ability to activate specific muscles, leading to muscle weakness and paralysis. ALS is a progressive disease that worsens over time, and often leads to death within three to five years from when symptoms first appear.
How is this drug used?
RELYVRIO is taken orally once daily for three weeks, and then the dosage can be increased to twice a day. It is taken by combining one packet in 8 ounces of room temperature water and stirring vigorously. RELYVRIO should be taken before a snack or meal. It can also be administered through a feeding tube.
Who participated in the clinical trials?
The FDA approved RELYVRIO based on safety and efficacy data from a single clinical trial (Study 1) of 137 adult patients with ALS. The trial was conducted at 25 sites in the United States.
How were the trials designed?
RELYVRIO was evaluated in a single clinical trial of 137 adult patients with ALS, who were randomly assigned to receive RELYVRIO or placebo for 24 weeks. Patients received RELYVRIO once daily for three weeks, and then increased the dose to twice daily. Neither the patients nor the healthcare providers knew which treatment was being given. The benefit was evaluated by measuring the ALS Functional Rating Scale-Revised (ALSFRS-R) and comparing the score at baseline compared to the score after 24 weeks.
After the study was completed, the overall average survival of patients who were originally chosen to receive RELYVRIO was compared to survival of patients originally chosen to receive placebo.
How were the trials designed?
RELYVRIO was evaluated in a single trial of 137 adult patients with ALS. The trial was a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study in adult patients with ALS. A total of 137 patients were randomized 2:1 to receive either RELYVRIO (N=89) or placebo (N=48) for 24 weeks. The primary endpoint was a comparison of the rate of reduction in the ALSFRS-R from baseline to Week 24.
There was a post hoc, long-term survival analysis which ascertained vital status on 136 of the 137 patients who were enrolled in Study 1. This exploratory analysis should be interpreted cautiously given the limitations of data collected outside of a controlled study.
DEMOGRAPHICS SNAPSHOT
Figure 1. Baseline Demographics by Sex (Study 1 Safety Population)
Source: Adapted from FDA Review
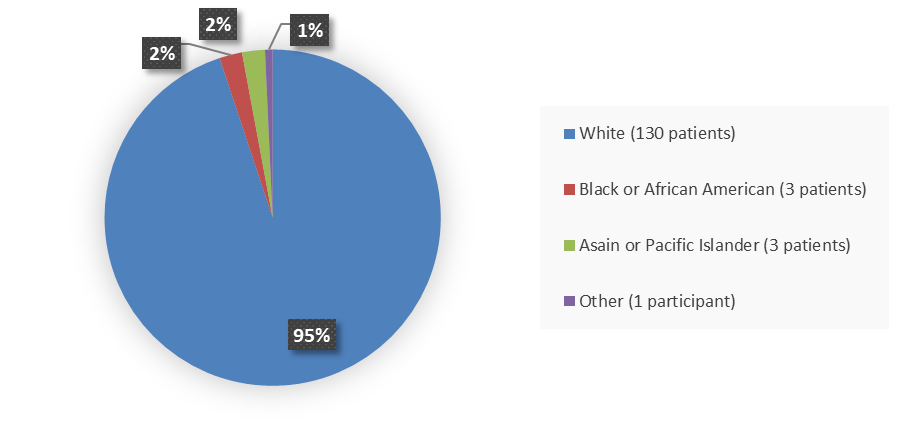
Figure 2. Baseline Demographics by Race (Study 1 Safety Population)
Source: Adapted from FDA Review
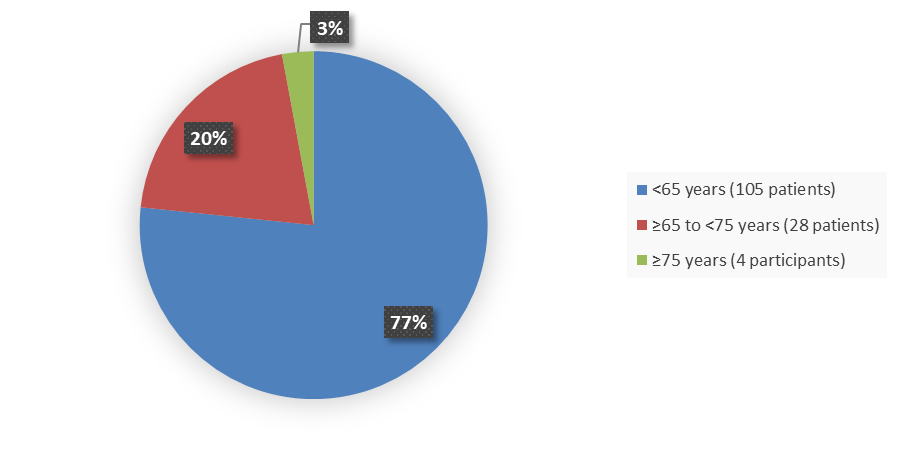
Figure 3. Baseline Demographics by Age (Study 1 Safety Population)
Source: Adapted from FDA Review
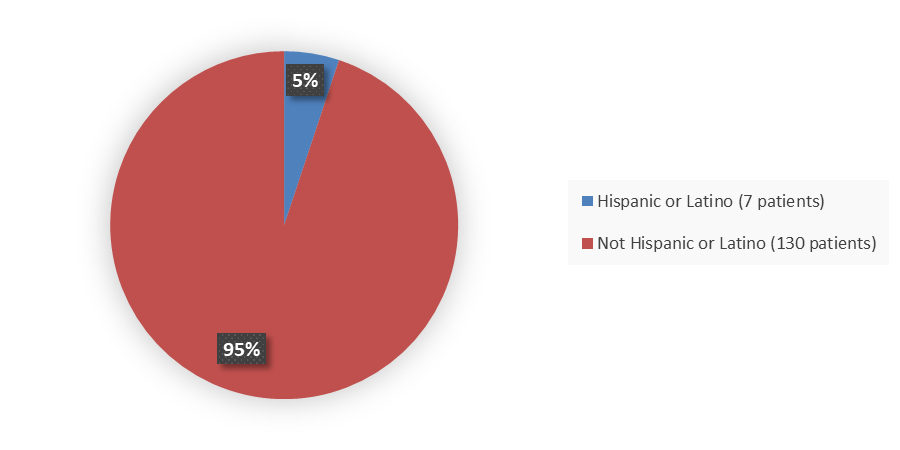
Figure 4. Baseline Demographics by Ethnicity (Study 1 Safety Population)
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes the demographics of patients who were randomized and dosed in Study 1 (Safety Population).
Table 1. Baseline Demographics of Patients in the Study 1 (Safety Population)
|
Demographic |
RELYVRIO |
Placebo |
Total |
|---|---|---|---|
|
Sex |
|||
|
Male |
61 (69) |
32 (67) |
93 (68) |
|
Female |
28 (31) |
16 (33) |
44 (32) |
|
Race |
|||
|
White |
84 (95) |
46 (96) |
130 (95) |
|
Asian |
2 (2) |
1 (2) |
3 (2) |
|
Black of African American |
2 (2) |
1 (2) |
3 (2) |
|
Other or missing |
1 (1) |
0 |
1 (1) |
|
Age group, years |
|||
|
<65 |
64 (72) |
41 (86) |
105 (77) |
|
≥65 to <75 |
22 (25) |
6 (12) |
28 (20) |
|
≥75 |
3 (3) |
1 (2) |
4 (3) |
|
Ethnicity |
|||
|
Hispanic or Latino |
6 (7) |
1 (2) |
7 (5) |
|
Not Hispanic or Latino |
83 (93) |
47 (98) |
130 (95) |
Source: Adapted from FDA Review
What are the benefits of this drug?
Patients treated with RELYVRIO experienced a slower rate of decline on a clinical assessment of daily functioning compared to patients receiving a placebo. Additionally, it was observed that some patients who originally received RELYVRIO lived longer when compared to those who originally received placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
RELYVRIO was evaluated in a 24-week, multicenter, randomized, double-blind, placebo controlled, parallel-group study in adult patients with ALS (Study 1). A total of 137 patients were randomized 2:1 to receive either RELYVRIO (N=89) or placebo (N=48) for 24 weeks. The primary endpoint was a comparison of the rate of reduction in the ALSFRS-R from baseline to Week 24.
There was a statistically significant difference in the rate of reduction in the ALSFRS-R total score from baseline to Week 24 in RELYVRIO-treated patients compared to placebo-treated patients (p=0.034). See Table 1.
Table 2. ALSFRS-R Total Score in Patients With ALS at Week 24 (mITT Population)
|
|
RELYVRIO |
Placebo |
|---|---|---|
|
ALSFRS-R total score at Week 24 LS mean (SE) |
29.06 (0.781) |
26.73 (0.975) |
|
Treatment difference (SE) |
2.32 (1.094) |
|
|
95% CI |
0.18, 4.47 |
|
|
P-value |
0.034 |
|
Source: RELYVRIO Prescribing Information
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI, confidence interval; LS, least squares; mITT, modified intent-to-treat; SE, standard error
In a post-hoc, long-term survival analysis, vital status was ascertained in 136 of 137 patients who were enrolled in Study 1. A longer median overall survival was observed in patients originally randomized to RELYVRIO compared to those originally randomized to placebo. This exploratory analysis should be interpreted cautiously given the limitations of data collected outside of a controlled study, which may be subject to confounding.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The observed effect of RELYVRIO was larger for males than females. Because of limited data, the ability to detect meaningful differences in how RELYVRIO worked is limited.
- Race: The number of patients of races other than White was small; therefore, differences in how RELYVRIO worked among races could not be determined.
- Age: The number of patients younger than 65 years of age was larger than participants 65 years of age and older; therefore, the ability to detect meaningful differences in how RELYVRIO worked is limited.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
In the Study 1 population, 95% of the randomized patients were White, 68% of patients were male, and 77% were younger than 65 years of age. The ability to interpret meaningful differences is limited due to the small number of patients and the imbalance in the number of patients in each subgroup. See Table 2 and Table 3.
Table 3. ALSFRS-R Total Score in Patients With ALS at Week 24 in Males and Females (mITT Population)
|
|
Male |
Female |
||
|---|---|---|---|---|
|
RELYVRIO |
Placebo |
RELYVRIO |
Placebo |
|
|
ALSFRS-R total score at Week 24 LS mean (SE) |
29.38 (1.01) |
26.13 (1.39) |
28.25 (1.55) |
27.94 (1.94) |
|
Treatment difference (SE) |
3.25 (1.34) |
0.31 (1.91) |
||
|
95% CI |
0.63, 5.88 |
-3.42, 4.05 |
||
|
P-value |
0.016 |
0.871 |
||
Source: Adapted from FDA Review
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI, confidence interval; LS, least squares; mITT, modified intent-to-treat; SE, standard error
Table 4 ALSFRS-R Total Score in Patients With ALS at Week 24 in Patients <65 and ≥65 Years of Age (mITT Population)
|
Parameter |
<65 Years |
≥65 Years |
||
|---|---|---|---|---|
|
RELYVRIO |
Placebo |
RELYVRIO |
Placebo |
|
|
ALSFRS-R total score at Week 24 LS mean (SE) |
29.06 (1.05) |
26.60 (1.26) |
28.90 (1.86) |
27.71 (3.10) |
|
Treatment difference (SE) |
2.46 (1.24) |
1.19 (2.57) |
||
|
95% CI |
0.03, 4.89 |
-3.86, 6.23 |
||
|
P-value |
0.048 |
0.645 |
||
Source: Adapted from FDA Review
Abbreviations: ALS, amyotrophic lateral sclerosis; ALSFRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI, confidence interval; LS, least squares; mITT, modified intent-to-treat; SE, standard error
What are the possible side effects?
The most common adverse reactions experienced with RELYVRIO were diarrhea, abdominal pain, nausea, and upper respiratory tract infection. RELYVRIO contains Tau ursodiol, a bile acid, which may cause worsening diarrhea in patients with disorders that interfere with bile acid circulation. These patients should consult with a specialist before taking RELYVRIO.
What are the possible side effects (results of trials used to assess safety)?
Table 4 lists the common adverse reactions that occurred in more than 5% of patients taking RELYVRIO and were at least 5% greater than in patients taking placebo in Study 1. Gastrointestinal-related adverse reactions occurred throughout the study but were more frequent during the first three weeks of treatment.
Table 5. Adverse Reactions Reported in More Than 5% of RELYVRIO-Treated Patients With ALS and at Least 5% Greater Than Placebo (Study 1)
|
Adverse Reaction |
RELYVRIO |
Placebo |
|---|---|---|
|
Diarrhea* |
25 |
19 |
|
Abdominal pain* |
21 |
13 |
|
Nausea |
18 |
13 |
|
Upper respiratory tract infection* |
18 |
10 |
|
Fatigue* |
12 |
6 |
|
Salivary hypersecretion |
11 |
2 |
Source: RELYVRIO Prescribing Information
*Adverse reaction is composed of several similar terms
Abbreviations: ALS, amyotrophic lateral sclerosis
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females, with slightly higher rates of some side effects in females.
- Race: The number of patients of races other than White was small; therefore, differences in the side effects of RELYVRIO among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age with the exception of abdominal pain which was more prevalent in patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The occurrence of adverse events was similar in males and females; however, there were slightly higher rates of some adverse events among females receiving RELYVRIO compared to males, including dizziness, dysarthria, abdominal pain, diarrhea, nausea, and vomiting. The sample size is small and conclusions may not be reliable. See Table 5.
Table 6. TEAE Comparisons in Male and Female Patients for AEs Occurring in Higher Percentage of AMX0035 Treated Patients Compared to Placebo
|
Preferred Terms |
Males |
Females |
|---|---|---|
|
Dizziness |
2 (3) |
7 (25) |
|
Dysarthria |
3 (5) |
4 (14) |
|
Abdominal pain |
12 (20) |
7 (25) |
|
Diarrhea |
17 (28) |
5 (18) |
|
Nausea |
2 (3) |
8 (29) |
|
Vomiting |
1 (2) |
3 (11) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event; TEAE, treatment-emergent adverse event
The occurrence of adverse events was similar in patients ages younger than 65 years and 65 years or older. The sample size is small and conclusions may not be reliable. SeeTable 7.
Table 7 TEAE Comparisons in Patients Ages <65 Years and ≥65 Years AEs Occurring in Higher Percentage of AMX0035 Treated Patients Compared to Placebo
|
Preferred Terms |
<65 Years |
≥65 Years |
|---|---|---|
|
Dizziness |
7 (11) |
2 (8) |
|
Dysarthria |
5 (8) |
2 (8) |
|
Abdominal pain |
9 (14) |
10 (40) |
|
Diarrhea |
15(23) |
7 (28) |
|
Nausea |
11 (17) |
5 (20) |
|
Vomiting |
4 (6) |
0 (0) |
Source: Adapted from FDA Review
Abbreviations: AE, adverse event; TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
LINK TO DRUG PACKAGE INSERT
https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/216660s000lbledt.pdf