Drug Trials Snapshots: NUZYRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the NUZYRA Package Insert for complete information.
NUZYRA (omadacycline)
nu-zahy-ruh
Paratek Pharmaceuticals, Inc.
Approval date: October 2, 2018
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
NUZYRA is an antibacterial medicine used to treat adult patients with community-acquired bacterial pneumonia (CABP).
Pneumonia is a type of lung infection. Community-acquired bacterial pneumonia (CABP) develops in people with limited or no contact with hospitals or health care centers.
How is this drug used?
NUZYRA is given by a healthcare professional using a needle placed in a vein (known as intravenous or IV infusion) over 60 minutes once a day for 7 to 14 days.
NUZYRA can also be taken as a tablet by mouth once a day for 7 to 14 days.
What are the benefits of this drug?
NUZYRA worked similarly to moxifloxacin (an antibacterial medication used to treat CABP) in improving signs and symptoms of pneumonia (cough, sputum production, chest pain, or difficulty breathing).
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients in Trial 1. The primary endpoint was the response to therapy at the early clinical response (ECR) assessment 72 to 120 hours after administration of the first dose of treatment.
| Table 2: Clinical Success 72-120 hours* in Trial 1 [Intent-to-Treat (ITT) Population] | |||
|---|---|---|---|
| Endpoint | NUZYRA n/N (%) | Moxifloxacin n/N (%) | Treatment Difference (95% CI**) |
| Clinical Success | 81.1% | 82.7% | ‑1.6 (‑7.1, 3.8) |
* Clinical Success at the early clinical response (ECR) timepoint, 72 to 120 hours after the first dose, was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) from baseline without deterioration in any of these symptoms, with no receipt of antibacterial treatment either as a rescue for CABP or as a treatment for other infections that may be effective for CABP, and no discontinuation of study treatment due to AE.
**95% confidence interval for the treatment difference
NUZYRA Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: NUZYRA worked similarly in men and women.
- Race: The majority of patients was White. Differences in how well the drug worked among races could not be determined because of the small number of patients of other races.
- Age: NUZYRA worked similarly in patients younger than and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The figure below summarizes efficacy results by sex, race, and age.
Table 3. Subgroup Efficacy Analyses of the Primary Endpoint (Clinical Success at ECR): ITT
| Subgroup | NUZYRA | moxifloxacin |
|---|---|---|
| Sex | ||
| Male | 174/208 (83.7) | 178/219 (81.3) |
| Female | 139/178 (78.1) | 143/169 (84.6) |
| Race | ||
| White | 288/356 (80.9) | 293/355 (82.5) |
| Black or African American | 8/11 (72.7) | 6/7 (85.7) |
| Asian | 16/17 (94.1) | 16/18 (88.9) |
| Other | 1/2 (50.0) | 6/8 (75.0) |
| Age | ||
| < 65 years | 197/234 (84.2) | 183/216 (84.7) |
| > 65 years | 116/152 (76.3) | 138/172 (80.2) |
FDA Review
What are the possible side effects?
All deaths in the trial occurred in patients older than 65 years of age and more commonly in patients treated with NUZYRA who were older than 65 years of age.
NUZYRA like other antibacterial medications called tetracyclines may cause serious side effects including
- permanently turning a child’s teeth yellow, gray, or brown from birth to 8 years of age
- slow bone growth
- diarrhea caused by an infection called clostridium difficile
The most common side effects are increased liver tests, increased blood pressure (hypertension), difficulty sleeping (insomnia), vomiting, constipation, and nausea.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions in adult patients with CABP in Trial 1 (safety population).
| Table 4: Adverse Reactions Occurring in > 2% of Patients Receiving NUZYRA in Trial 1 | ||
|---|---|---|
| Adverse Reaction | NUZYRA (N = 382) |
Moxifloxacin (N = 388) |
| Alanine aminotransferase increased | 3.7 | 4.6 |
| Hypertension | 3.4 | 2.8 |
| Gamma-glutamyl transferase increased | 2.6 | 2.1 |
| Insomnia | 2.6 | 2.1 |
| Vomiting | 2.6 | 1.5 |
| Constipation | 2.4 | 1.5 |
| Nausea | 2.4 | 5.4 |
| Aspartate aminotransferase increased | 2.1 | 3.6 |
| Headache | 2.1 | 1.3 |
NUZYRA Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The majority of patients was White. The occurrence of side effects among races could not be determined because of the small number of patients of other races.
- Age: The occurrence of side effects was similar among patients younger than and older than 65 years of age except for the death cases which all occurred in patients older than 65 years.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The table below summarizes the occurrence of the most common adverse reaction, increased alanine aminotransferase, by subgroup.
Table 5. Subgroup Analysis of Increased Alanine Aminotransferase (safety population)
| Demographic Characteristic | NUZYRA n/N (%) |
moxifloxacin n/N (%) |
|---|---|---|
| Sex | ||
| Men | 8/205 (4) | 15/219 (7) |
| Women | 6/177 (3) | 3/169 (2) |
| Race | ||
| White | 14/353 (4) | 17/355 (5) |
| Black or African American | 0/11 (0) | 0/7 (0) |
| Asian | 0/17 (0) | 0/18 (0) |
| American Indian or Alaska Native | 0/0 (0) | 1/2 (50) |
| Other | 0/1 (0) | 0/6 (0) |
| Age Group | ||
| < 65 years | 9/232 (4) | 11/216 (5) |
| > 65 years | 5/149 (3) | 7/172 (4) |
Clinical Trial Data
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved NUZYRA based on evidence from one clinical trial (Trial 1 /NCT02531438) of 774 patients with community acquired bacterial pneumonia (CABP).
The trial was conducted at 86 sites in, Asia, Europe, Israel, Latin America, South Africa, and the United States. Demographics of the patients who provided data for evaluation of side effects (safety population) are presented in Table 7, under the MORE INFO section. The population that provided data for benefits of NUZYRA (efficacy population) is presented below.
Figure 1 summarizes how many men and women were in the clinical trial.
Figure 1. Baseline Demographics by Sex (efficacy population)
FDA Review
Figure 2 summarizes the percentage of patients by race in the clinical trial.
Figure 2. Baseline Demographics by Race (efficacy population)
*Other includes American Indian or Alaska Native
FDA Review
Table 1. Demographics of Trial by Race (efficacy population)
| Race | Number of Patients | Percentage of Patients |
|---|---|---|
| White | 711 | 92% |
| Black or African American | 18 | 2% |
| Asian | 35 | 5% |
| American Indian or Alaska Native | 2 | Less than 1% |
| Other | 8 | 1% |
FDA Review
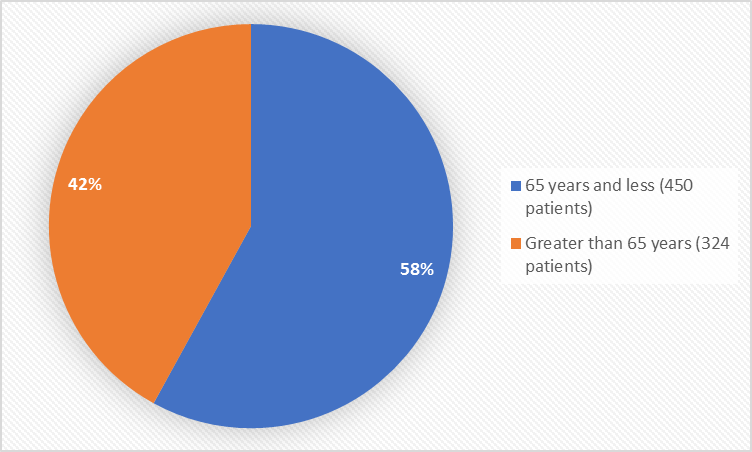
Figure 3. Baseline Demographics by Age
FDA Review
Who participated in the trials?
The table below summarizes demographics of the patients in the clinical trial.
Table 6. Demographic Characteristics of Safety Population (Trial 1)
| Demographic Characteristics | NUZYRA (N = 382) |
moxifloxacin (N = 388) |
Total (N = 770) |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 205 (54) | 219 (56) | 424 (55) |
| Women | 177 (46) | 169 (44) | 346 (45) |
| Race, n (%) | |||
| White | 353 (92) | 355 (91) | 708 (92) |
| Black or African American | 11 (3) | 7 (2) | 18 (2) |
| Asian | 17 (4) | 18 (5) | 35 (5) |
| American Indian or Alaska Native | 0 (0) | 2 (< 1) | 2 (< 1) |
| Other | 1 (< 1) | 6 (2) | 7 (1) |
| Age (years) | |||
| Mean | 61 (15) | 62 (15) | 62 (15) |
| Min, max | 19, 97 | 19, 94 | 19, 97 |
| Age Group, n (%) | |||
| 18-65 | 232 (61) | 216 (56) | 448 (58) |
| > 65-75 | 76 (20) | 89 (23) | 165 (21) |
| > 75 | 74 (19) | 83 (21) | 157 (20) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 8 (2) | 14 (4) | 22 (3) |
| Not Hispanic or Latino | 370 (97) | 370 (95) | 740 (96) |
| Not Reported/unknown | 4 (1) | 4 (1) | 8 (1) |
| Region, n (%) | |||
| Europe | 247 | 248 | 495 (64) |
| United States | 1 | 2 | 3 (< 1) |
| Rest of the World | 134 | 138 | 272 (35) |
Clinical Trial Data
Table 7. Demographic Characteristics of Intent-to-Treat (ITT) Population (Trial 1)
| Demographic Characteristics | NUZYRA (N = 386) |
moxifloxacin (N = 388) |
Total (N = 774) |
|---|---|---|---|
| Sex, n (%) | |||
| Men | 208 (54) | 219 (56) | 427 (55) |
| Women | 178 (46) | 169 (44) | 347 (45) |
| Race, n (%) | |||
| White | 356 (92) | 355 (91) | 711 (92) |
| Black or African American | 11 (3) | 7 (2) | 18 (2) |
| Asian | 17 (4) | 18 (5) | 35 (5) |
| American Indian or Alaska Native | 0 (0) | 2 (< 1) | 2 (< 1) |
| Other | 2 (< 1) | 6 (2) | 8 (1) |
| Age (years) | |||
| Mean | 61 (15) | 62 (15) | 62 (15) |
| Min, max | 19, 97 | 19, 94 | 19, 97 |
| Age Group, n (%) | |||
| 18-65 | 234 (61) | 216 (56) | 450 (58) |
| > 65-75 | 152 (39) | 172 (44) | 324 (42) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 10 (3) | 14 (4) | 24 (3) |
| Not Hispanic or Latino | 372 (96) | 370 (95) | 742 (96) |
| Not Reported/unknown | 4 (1) | 4 (1) | 8 (1) |
| Region, n (%) | |||
| Europe | 251 (65) | 248 (64) | 499 (64) |
| United States | 1 (< 1) | 2 (< 1) | 3 (< 1) |
| Rest of the World | 134 (35) | 138 (36) | 272 (35) |
FDA Review
How were the trials designed?
The benefit and side effects of NUZYRA were evaluated in one clinical trial of adult patients 18 – 97 years of age with community acquired bacterial pneumonia (CABP). Patients were assigned to receive NUZYRA every 12 hours for 2 doses on Day 1 followed by daily intravenous doses, or moxifloxacin (a prescription drug used to treat bacterial infections) for 7 to 14 days. Patients could switch to medication by mouth after 3 days of treatment. Neither the patients nor the health care providers knew which treatment was being given until after the trial was completed. The benefit of NUZYRA was assessed based on the response to treatment 72 to 120 hours after the first dose of treatment, comparing patients in the NUZYRA and moxifloxacin groups. A positive response was improvement from baseline on at least 2 out of 4 symptoms (cough, coughing up and spitting out phlegm, pain in the chest when breathing in or out, and difficulty breathing) with no worsening of other symptoms.
How were the trials designed?
The efficacy and safety of NUZYRA were evaluated in one clinical trial.
Trial 1 was a randomized, double blind, double-dummy, non-inferiority trial comparing NUZYRA and moxifloxacin for the treatment of CABP, in adult patients. Patients were randomized to receive NUZYRA 10 mg intravenously every 12 hours for 2 doses on Day 1, followed by 100 mg intravenously or 300 mg once a day or moxifloxacin 400 mg intravenously or orally once a day for 7 to 14 days. The primary efficacy endpoint was clinical success at 72 – 120 hours. Clinical success was defined as survival with improvement in at least two of four symptoms (cough, sputum production, chest pain, dyspnea) from baseline without deterioration in any of these symptoms, with no receipt of antibacterial treatment either as a rescue for CABP or as a treatment for other infections that may be effective for CABP, and no discontinuation of study treatment due to adverse events.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.
PRESCRIBING INFORMATION