Drug Trials Snapshots: MODEYSO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the MODEYSO Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
MODEYSO (dordaviprone)
(moh-DAY-soh)
Jazz Pharmaceuticals
Approval date: August 06, 2025
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
MODEYSO is a prescription drug used for the treatment of adult and pediatric patients 1 year of age and older with diffuse midline glioma harboring an H3 K27M mutation with progressive disease following prior therapy.
How is this drug used?
MODEYSO is administered as one or more capsules taken by mouth once weekly without food. Capsule contents can be opened and mixed with appropriate liquids (water, apple juice, lemonade or sports drink) and administered orally for the patients unable to swallow capsules whole.
Who participated in the clinical trials?
The FDA granted accelerated approval of MODEYSO based on evidence from five clinical trials (ONC006 [NCT02525692], ONC013 [NCT03295396], ONC014 [NCT03416530], ONC016 [NCT05392374], and ONC018 [NCT03134131]). These clinical trials enrolled both adults and children with glioma and were conducted at 68 sites in the United States. All five trials contributed to the efficacy analysis, while four of them (all except ONC016) were also used to evaluate safety.
The efficacy population included 50 adult and pediatric patients with progressive and measurable diffuse midline glioma harboring an H3 K27M mutation. The median age was 31 years (range: 9 to 70), and 6% were younger than 17 years of age. Of these patients, 46% were female. By race and ethnicity, 80% were White, 6% Black or African American, 2% Asian, 10% identified as other races, 2% were unknown, and 8% were Hispanic or Latino.
The safety population included 376 adult and pediatric patients who received MODEYSO at the recommended weight-based dose. The median age was 23 years (range: 3 to 80); 30% were 2 to 11 years old, 11% were 12 to 17 years old, 55% were 18 to 64 years old, and 3.7% aged 65 years or older. Of these patients, 52% were female. By race and ethnicity, 74% were White, 9% Black or African American, 4% Asian, 2.9% other or multiple races, 10% were unknown, and 13% were Hispanic or Latino.
How were the trials designed?
MODEYSO was evaluated in 50 adult and pediatric patients with H3 K27M-mutant diffuse midline glioma across five clinical trials. The benefit of MODEYSO was evaluated by measuring the percentage of patients who had complete, partial, or minor responses (overall response rate or ORR) assessed by blinded independent central review (BICR) according to brain tumor-specific response criteria, and by measuring the duration of that decrease in tumor size (duration of response or DOR).
The side effects of MODEYSO were evaluated in 376 adult and pediatric patients with glioma across four clinical trials.
How were the trials designed?
The efficacy of MODEYSO was evaluated in adult and pediatric patients with glioma across five open label, non-randomized clinical trials conducted in the United States (ONC006, ONC013, ONC014, ONC016, and ONC018). Pre-specified criteria were defined to establish an integrated efficacy population; eligible patients were required to have received single-agent MODEYSO, have diffuse midline glioma harboring an H3 K27M mutation with progressive and measurable disease per Response Assessment in Neuro Oncology-High Grade Glioma (RANO-HGG) criteria, be ≥90 days post radiation therapy, have adequate washout from prior anticancer therapies, have a Karnofsky Performance Status/Lansky Performance Status (KPS/LPS) score ≥60, and have stable or decreasing corticosteroid use. Patients with diffuse intrinsic pontine glioma (DIPG), primary spinal tumors, atypical histologies, or cerebrospinal fluid dissemination were excluded.
The integrated efficacy population included 50 patients who met these criteria. The major efficacy outcome measure was ORR assessed by BICR according to Response Assessment in Neuro Oncology (RANO) 2.0 criteria. Additional efficacy outcome measures were BICR assessed ORR according to RANO-HGG criteria and Response Assessment in Neuro Oncology-Low Grade Glioma (RANO-LGG) criteria, duration of response, and time to response.
The safety of MODEYSO was evaluated in 376 adult and pediatric patients with glioma across four open label, non-randomized clinical trials conducted in the United States (ONC006, ONC013, ONC014, and ONC018). This pooled safety population reflects exposure to MODEYSO at the recommended weight-based dose taken until disease progression or unacceptable toxicity.
DEMOGRAPHICS SNAPSHOT
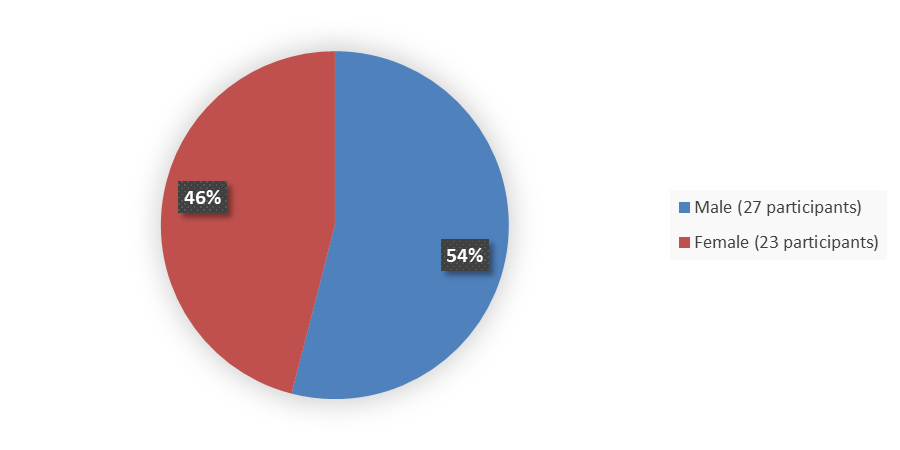
The efficacy population for this application included 50 patients with H3 K27M mutant diffuse midline glioma. Figure 1 summarizes the percentage of patients by sex in the efficacy population.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
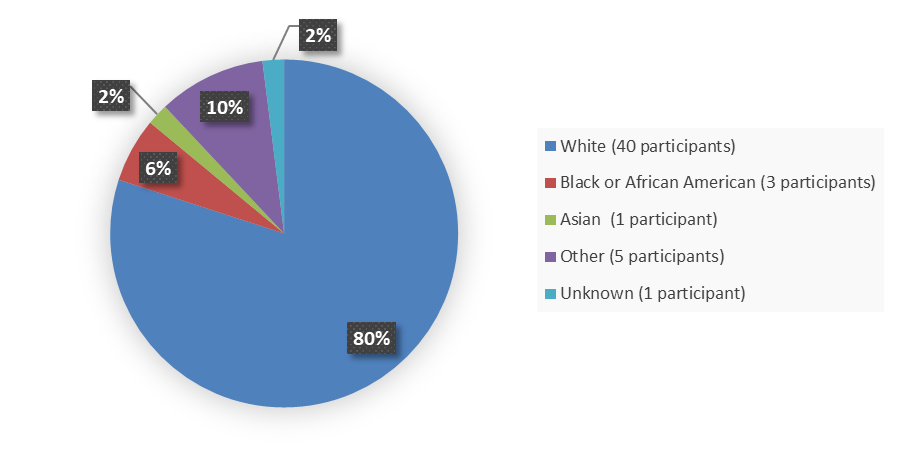
Figure 2 summarizes the percentage of patients by race in the efficacy population.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
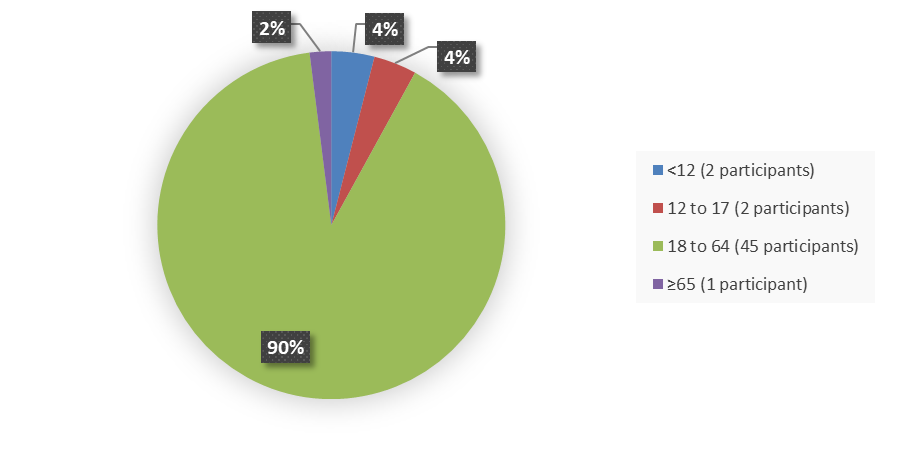
Figure 3 summarizes the percentage of patients by age in the efficacy population.
Figure 3. Baseline Demographics by Age, Efficacy Population
Source: Adapted from FDA Review
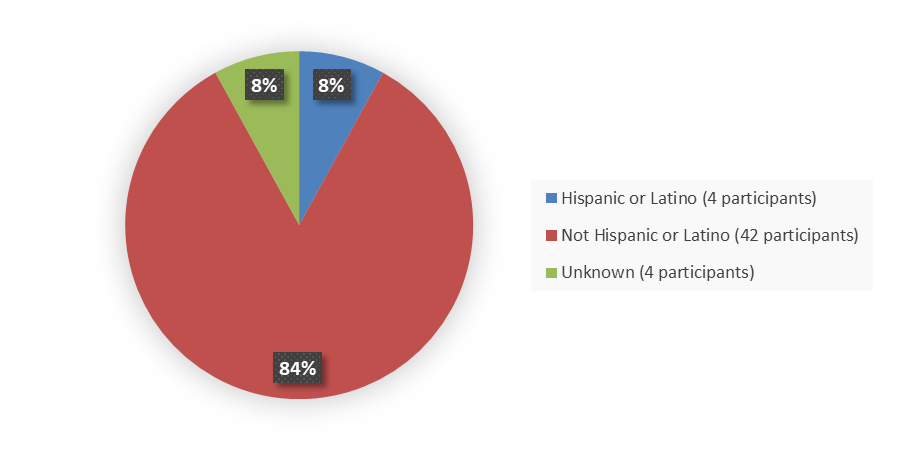
Figure 4 summarizes the percentage of patients by ethnicity in the efficacy population.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
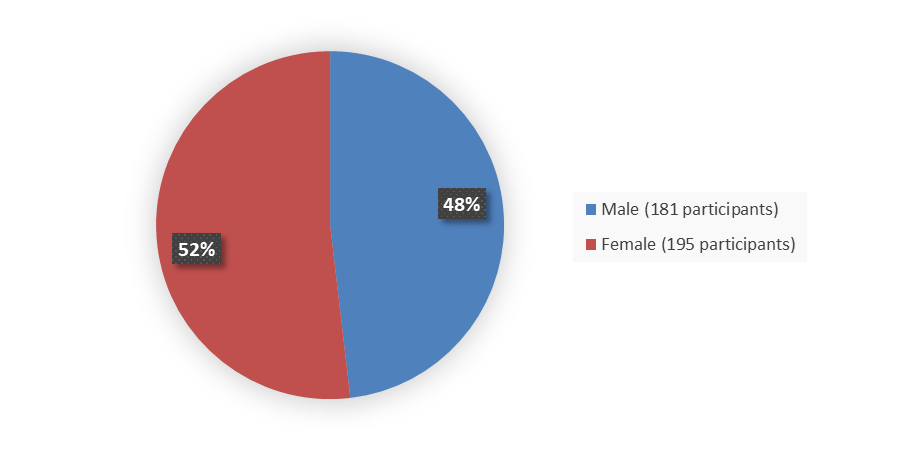
The safety population for this application included 376 patients with glioma. Figure 5 summarizes how many male and female patients were included in the safety population.
Figure 5. Baseline Demographics by Sex, Safety Population
Source: Adapted from FDA Review
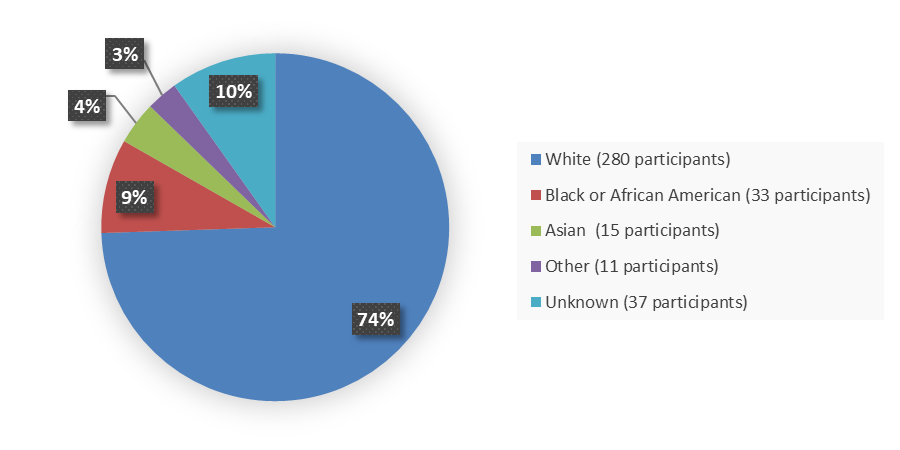
Figure 6 summarizes the percentage of patients by race in the safety population.
Figure 6. Baseline Demographics by Race, Safety Population
Source: Adapted from FDA Review
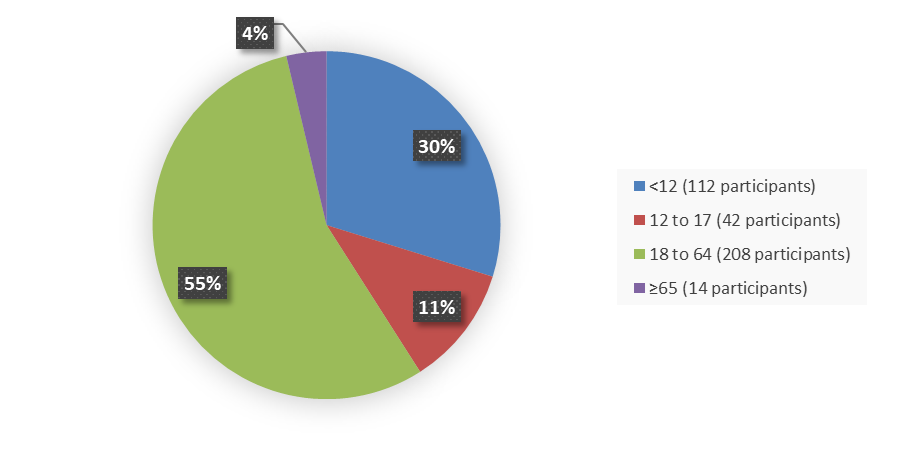
Figure 7 summarizes the percentage of patients by age in the safety population.
Figure 7. Baseline Demographics by Age, Safety Population
Source: Adapted from FDA Review
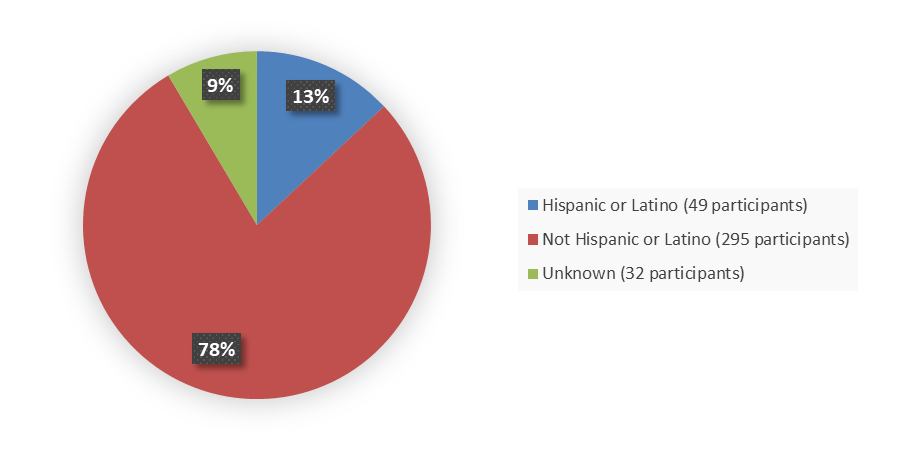
Figure 8 summarizes the percentage of patients by ethnicity in the safety population.
Figure 8. Baseline Demographics by Ethnicity, Safety Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 summarizes baseline demographics for the efficacy and safety populations.
Table 1. Baseline Demographics in Efficacy and Safety Populations
| Demographic | Efficacy Population N=50 n (%) | Safety Population N=376 n (%) |
|---|---|---|
| Age, years | ||
| <12 | 2 (4) | 112 (30) |
| 12 to 17 | 2 (4) | 42 (11) |
| 18 to 64 | 45 (90) | 208 (55) |
| ≥65 | 1 (2) | 14 (4) |
| Sex | ||
| Female | 23 (46) | 195 (52) |
| Male | 27 (54) | 181 (48) |
| Race | ||
| White | 40 (80) | 280 (74) |
| Black or African American | 3 (6) | 33 (9) |
| Asian | 1 (2) | 15 (4) |
| Other | 5 (10) | 11 (2.9) |
| Unknown | 1 (2) | 37 (10) |
| Ethnicity | ||
| Hispanic or Latino | 4 (8) | 49 (13) |
| Not Hispanic or Latino | 42 (84) | 295 (78) |
| Unknown | 4 (8) | 32 (9) |
Source: Adapted from FDA Review
What are the benefits of this drug?
MODEYSO was approved under FDA’s accelerated approval program, which provides earlier patient access to a promising new drug while the company continues to conduct clinical trials to confirm that the drug works well.
In the efficacy population, 22% of patients with H3 K27M-mutant diffuse midline glioma experienced partial or minor shrinkage of their tumors; of these patients, 73% had shrinkage of their tumor that lasted more than six months. One additional patient also had tumor shrinkage when evaluated with an assessment method that factored in both corticosteroid use and performance status.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 summarizes the efficacy results.
In the efficacy population, the overall response rate was 22% (95% CI: 12, 36) according to the RANO 2.0 criteria assessed by BICR; the median DOR was 10.3 months. Among responders, the median time to response was 3.6 months (range 1.6, 15.6).
Table 2. Efficacy Results for Patients With Diffuse Midline Glioma Harboring an H3 K27M Mutation, Efficacy Population
| Efficacy Parameter | MODEYSO N=50 |
|---|---|
| Overall response rate, % (95% CI)a | 22 (12, 36) |
| Partial response, % | 16 |
| Minor response, % | 6 |
| Duration of response | N=11 |
| Median (95% CI)b, months | 10.3 (7.3, 15.2) |
| % with observed DOR ≥6 monthsc | 73 |
| % with observed DOR ≥12 monthsc | 27 |
Source: Adapted from MODEYSO Prescribing Information
a Confirmed overall response rate assessed by BICR; CI based on Clopper-Pearson method.
b Based on Kaplan-Meier estimate.
c Based on observed time.
Abbreviations: BICR, blinded independent central review; CI, confidence interval; DOR, duration of response
There was one additional responder based on the integrated response assessment, which takes into account corticosteroid use and performance status. Based on BICR-assessed RANO-HGG criteria (n=50), the ORR was 20% (95% CI: 10, 34), with one complete and nine partial responses. Based on BICR-assessed RANO-LGG criteria (n=50), the ORR was 20% (95% CI: 10, 34), with five partial and five minor responses.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: MODEYSO worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how MODEYSO worked among races could not be determined.
- Age: There were four patients younger than 18 years and one patient older than 65 years of age in the efficacy population; therefore, differences in how MODEYSO worked among age groups could not be determined.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 3 summarizes efficacy results by sex, age, and race subgroups, respectively.
Table 3. Efficacy Results by Subgroup, Efficacy Population
| Subgroup | Responders/n | ORR, % (95% CI) |
|---|---|---|
| Age, years | ||
| <65 | 11/49 | 22 (12, 37) |
| ≥65 | 0/1 | 0 |
| Sex | ||
| Male | 6/27 | 22 (9, 42) |
| Female | 5/23 | 22 (7, 44) |
| Race | ||
| White | 9/40 | 23 (11, 38) |
| Black or African American | 0/3 | 0 |
| Asian | 0/1 | 0 |
| American Indian or Alaska Native | 0/1 | 0 |
| Other or Unknown | 2/5 | 40 (5, 85) |
Source: Adapted from FDA Assessment Aid
Abbreviation: CI, confidence interval; ORR, overall response rate
What are the possible side effects?
MODEYSO may cause serious side effects, including allergic reactions, changes in heart rhythm (QTc interval prolongation), or harm to an unborn baby.
The most common side effects of MODEYSO are tiredness (fatigue); headache; vomiting; nausea; and muscle, joint, and bone pain.
The most common abnormal blood tests include decreased white blood cells, decreased red blood cells, decreased calcium, and increased liver enzymes.
What are the possible side effects (results of trials used to assess safety)?
The safety of MODEYSO was evaluated in 376 adult and pediatric patients with glioma across four clinical trials (ONC006, ONC013, ONC014, and ONC018). Table 4 summarizes adverse reactions that occurred in at least 10% of patients treated with MODEYSO in the safety population.
Table 4. Adverse Reactions (≥10%) in Patients With Glioma Who Received MODEYSO, Safety Population
| Adverse Reaction | MODEYSO, N=376 | |
|---|---|---|
| All Grades % | Grade 3 or 4 % | |
| General disorders | ||
| Fatiguea | 34 | 3.2 |
| Gait disturbance | 16 | 3.7 |
| Nervous system disorders | ||
| Headacheb | 32 | 4.3 |
| Cranial nerve disordersc | 16 | 1.3 |
| Hemiparesis | 15 | 4.5 |
| Dysarthria | 13 | 2.7 |
| Dizziness | 13 | 0.5 |
| Ataxia | 10 | 1.3 |
| Gastrointestinal disorders | ||
| Vomiting | 24 | 2.7 |
| Nausea | 24 | 0.8 |
| Dysphagia | 13 | 2.1 |
| Constipation | 11 | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal paind | 20 | 2.9 |
| Muscular weakness | 13 | 4.5 |
| Metabolism and nutrition disorders | ||
| Hyperglycemia | 12 | 0.8 |
| Infections and infestations | ||
| Rashe | 11 | 0.8 |
Source: Adapted from MODEYSO Prescribing Information
a Includes asthenia.
b Includes head discomfort and sinus headache.
c Includes accessory nerve disorder, auditory nerve disorder, facial nerve disorder, facial paralysis, facial paresis, glossopharyngeal nerve disorder, hypoglossal nerve disorder, IIIrd nerve disorder, IIIrd nerve paralysis, IVth nerve disorder, IVth nerve paralysis, tongue paralysis, trigeminal nerve disorder, trigeminal neuralgia, VIth nerve disorder, VIth nerve paralysis, and VIth nerve paresis.
d Includes back pain, pain in extremity, arthralgia, neck pain, non-cardiac chest pain, myalgia, bone pain, musculoskeletal chest pain, musculoskeletal stiffness, and spinal pain.
e Includes dermatitis, dermatitis acneiform, dermatitis bullous, eczema, erythema multiforme, rash, erythematous, rash macular, rash maculo-papular, rash popular, rash pruritic, and rash pustular.
Table 5 summarizes select laboratory abnormalities that occurred in at least 10% of patients treated with MODEYSO in the safety population.
Table 5. Select Laboratory Abnormalities (≥10%) That Worsened From Baseline in Patients With Glioma Who Received MODEYSO, Safety Population
| Laboratory Abnormalitya | MODEYSOb | |
|---|---|---|
| All Grades % | Grade 3 or 4 % | |
| Chemistry | ||
| Alanine aminotransferase increased | 28 | 2.4 |
| Aspartate aminotransferase increased | 22 | 0.9 |
| Calcium decreased | 20 | 2.7 |
| Sodium decreased | 14 | 0.3 |
| Potassium decreased | 13 | 0.3 |
| Glucose decreased | 11 | 0 |
| Alkaline phosphatase increased | 11 | 0.3 |
| Hematology | ||
| Hemoglobin decreased | 25 | 0.6 |
| Neutrophils decreased | 24 | 1.5 |
| Lymphocytes decreased | 19 | 7 |
Source: Adapted from MODEYSO Prescribing Information
a Severity as defined by the National Cancer Institute CTCAE Version 5.0.
b The denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available, which ranged from 325 to 330 patients.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar across age groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 6, Table 7, and Table 8 summarize adverse reactions that occurred by sex, age, and race subgroups, respectively.
Table 6. Side Effects by Sex for Patients With Glioma Who Received MODEYSO, Safety Population
| Side Effect | Males N=181 % | Females N=195 % |
|---|---|---|
| TEAEs | 93 | 92 |
| Grade ≥3 TEAEs | 46 | 52 |
| Serious TEAEs | 34 | 32 |
| Fatal TEAEs | 9 | 8 |
Source: Adapted from FDA Review
Abbreviation: TEAE, treatment-emergent adverse event
Table 7. Side Effects by Age for Patients With Glioma Who Received MODEYSO, Safety Population
| Side Effect | <12 Years N=112 % | 12 to 17 Years N=42 % | 18 to 64 Years N=208 % | ≥65 Years N=14 % |
|---|---|---|---|---|
| TEAEs | 96 | 93 | 93 | 71 |
| Grade ≥3 TEAEs | 61 | 57 | 45 | 0 |
| Serious TEAEs | 38 | 38 | 31 | 0 |
| Fatal TEAEs | 15 | 10 | 6 | 0 |
Source: Adapted from FDA Review
Abbreviation: TEAE, treatment-emergent adverse event
Table 8. Side Effects by Race for Patients With Glioma Who Received MODEYSO, Safety Population
| Side Effect | White N=280 % | Black or African American N=33 % | Asian N=15 % | Other N=11 % | Unknown N=37 % |
|---|---|---|---|---|---|
| TEAEs | 93 | 94 | 93 | 100 | 86 |
| Grade ≥3 TEAEs | 50 | 55 | 47 | 45 | 43 |
| Serious TEAEs | 33 | 42 | 13 | 36 | 30 |
| Fatal TEAEs | 8 | 18 | 7 | 0 | 11 |
Source: Adapted from FDA Review
Abbreviation: TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.