Drug Trials Snapshots: IMJUDO
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the IMJUDO Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
IMJUDO (tremelimimab-actl)
im-JEW-doh
AstraZeneca UK LLC.
Original Approval date: October 21, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
IMJUDO is a cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blocking antibody indicated in combination with durvalumab, for the treatment of adult patients with unresectable hepatocellular carcinoma.
How is this drug used?
IMJUDO is given as an intravenous infusion and given as a single dose in combination with durvalumab.
Who participated in the clinical trials?
The FDA approved IMJUDO plus durvalumab based on evidence from one clinical trial (HIMALAYA trial, NCT03298451) that enrolled 782 patients with unresectable liver cancer. The trial was conducted at 175 clinical centers in 16 countries across regions of North and South America, Eastern and Western Europe, and Asia.
How were the trials designed?
The trial enrolled patients with a type of liver cancer that cannot be removed by surgery (unresectable hepatocellular carcinoma) whose cancer was not previously treated with prescription medicine. The enrolled patients received either IMJUDO as one dose combined with durvalumab or received sorafenib until disease progressed or intolerable side effects. The benefit of IMJUDO plus durvalumab was measured by overall survival.
How were the trials designed?
The benefit and side effects of IMJUDO taken in combination with durvalumab were evaluated in one clinical trial of patients with previously untreated hepatocellular carcinoma that spread to other parts of the body or that could not be removed by surgery.
In the trial, patients were randomized to receive IMJUDO plus durvalumab or sorafenib. The treatment continued until the disease progressed, the side effects became too toxic, or the patient decided to discontinue the study.
The benefit of IMJUDO was evaluated by measuring survival between patients who received IMJUDO plus durvalumab or sorafenib.
DEMOGRAPHICS SNAPSHOT
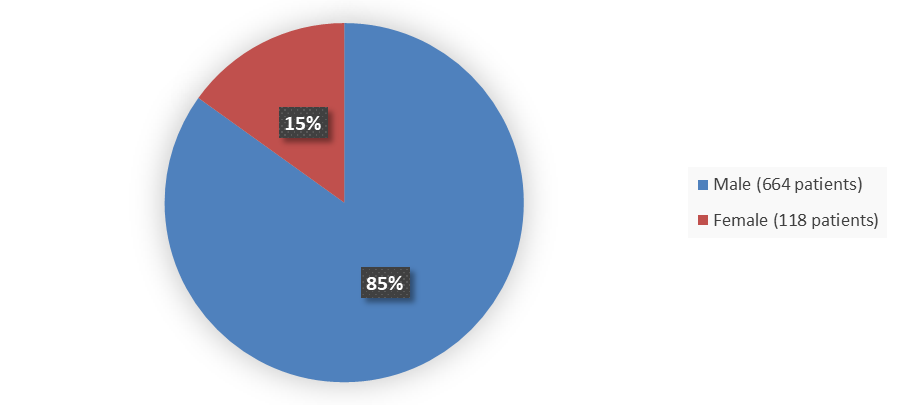
Figure 1. Baseline Demographics by Sex (Efficacy Population)
Source: Adapted from FDA Review
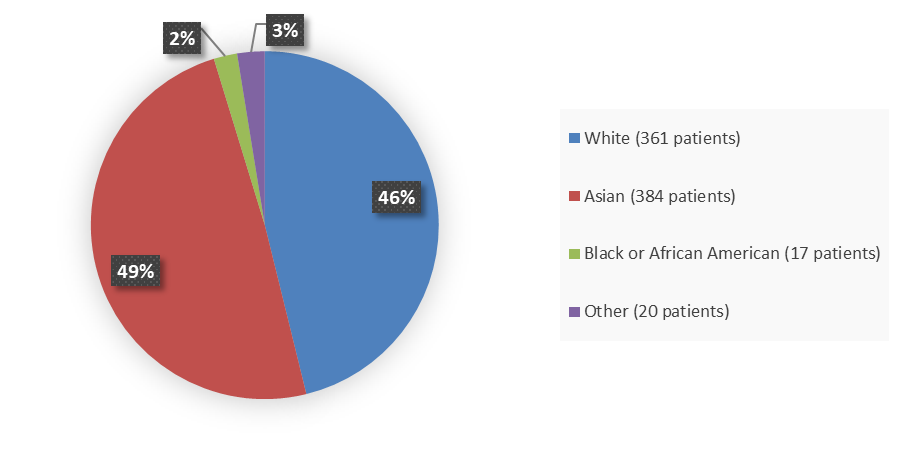
Figure 2. Baseline Demographics by Race (Efficacy Population)
Source: Adapted from FDA Review
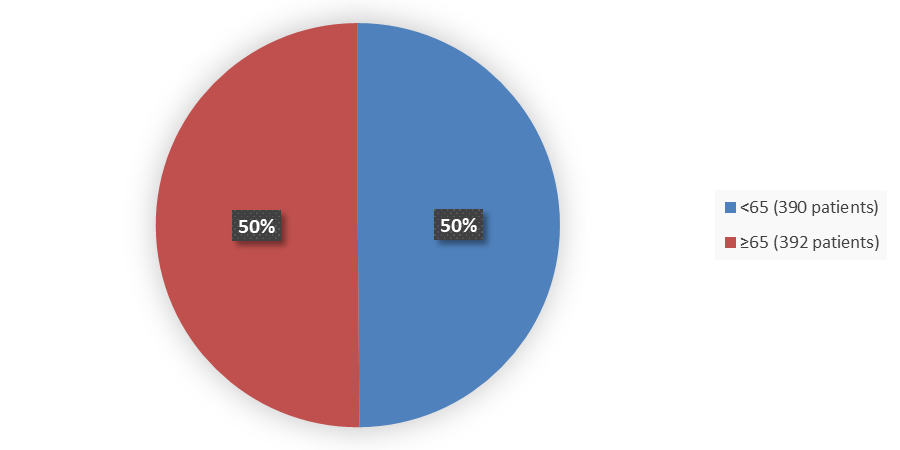
Figure 3 summarizes how many patients by age were enrolled in the clinical trial used to evaluate the efficacy of IMJUDO.
Figure 3. Baseline Demographics by Age (Efficacy Population)
Source: Adapted from FDA Review
Efficacy population includes patients treated with IMJUDO + durvalumab and patients treated with sorafenib on the HIMALAYA trial
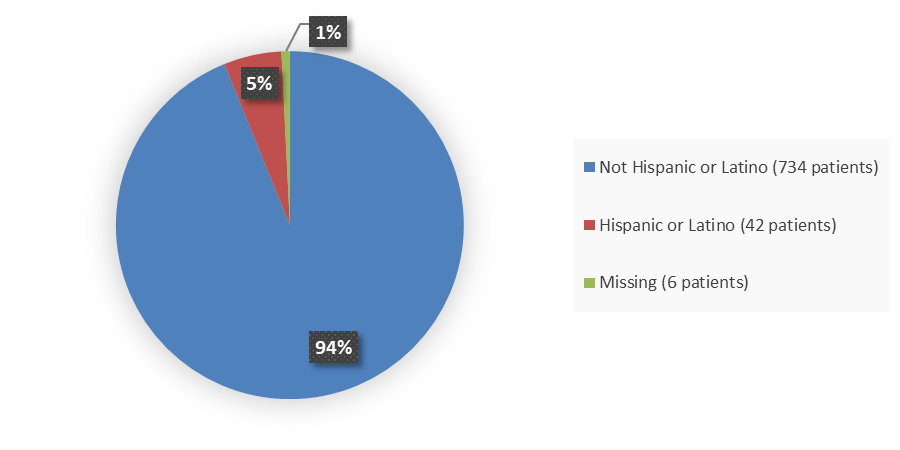
Figure 4. Baseline Demographics by Ethnicity (Efficacy Population)
Source: Adapted from FDA Review
Efficacy population includes patients treated with IMJUDO + durvalumab and patients treated with sorafenib on the HIMALAYA trial
Who participated in the trials?
Demographics for patients in the efficacy and safety trial populations are detailed in Table 1.
Table 1. Trial Demographics (Efficacy Population)
|
Demographic |
IMJUDO + |
|
|
|---|---|---|---|
|
Age group, years |
|||
|
<65 |
195 (49.6) |
195 (50.1) |
390 (49.9) |
|
≥65 |
198 (50.4) |
194 (49.9) |
392 (50.1) |
|
Sex |
|||
|
Male |
327 (83.2) |
337 (86.6) |
664 (84.9) |
|
Female |
66 (16.8) |
52 (13.4) |
118 (15.1) |
|
Race |
|||
|
White |
182 (46.3) |
179 (46.0) |
361 (46.2) |
|
Black or African American |
7 (1.8) |
10 (2.6) |
17 (2.2) |
|
Asian |
195 (49.6) |
189 (48.6) |
384 (49.1) |
|
Native Hawaiian or Other Pacific Islander |
1 (0.3) |
0 |
1 (0.1) |
|
Other |
7 (1.8) |
5 (1.3) |
12 (1.5) |
|
Missing |
1 (0.3) |
6 (1.5) |
7 (0.9) |
|
Ethnic group |
|||
|
Hispanic or Latino |
21 (5.3) |
21 (5.4) |
42 (5.4) |
|
Not Hispanic or Latino |
372 (94.7) |
362 (93.1) |
734 (93.9) |
|
Missing |
0 |
6 (1.5) |
6 (0.8) |
|
Region group |
|||
|
Asia (except Japan) |
156 (39.7) |
156 (40.1) |
312 (39.9) |
|
Rest of world (includes Japan) |
237 (60.3) |
233 (59.9) |
470 (60.1) |
Sources: Adapted from FDA Review
Efficacy population includes patients treated with IMJUDO + durvalumab and patients treated with Sorafenib IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: N, number of patients in treatment group; n, number of patients meeting criteria
What are the benefits of this drug?
In the trial, IMJUDO plus durvalumab lowered the risk of death by 22%, and 20% of people saw their tumor partially or fully shrink.
What are the benefits of this drug (results of trials used to assess efficacy)?
The results of the clinical trial that was used to assess efficacy are detailed in Table 2. The major efficacy outcome measure was overall survival. Additional efficacy outcome measures were progression free survival, overall response rate, and duration of response.
Table 2. Summary of Efficacy (Efficacy Population)
|
Efficacy Parameter |
IMJUDO + |
|
|---|---|---|
|
Overall survival |
||
|
Number of patient deaths, n (%) |
262 (66.7) |
293 (75.3) |
|
Median overall survival, months (95% CI) |
16.4 (14.2, 19.6) |
13.8 (12.3, 16.1) |
|
HR (95% CI) 1 |
0.78 (0.66, 0.92) |
|
|
p-value2,3 |
0.0035 |
|
|
Progression free survival (per investigator) |
||
|
Number of patients with event, n (%) |
335 (85.2) |
327 (84.1) |
|
Median PFS, months (95% CI) |
3.8 (3.7, 5.3) |
4.1 (3.7, 5.5) |
|
HR (95% CI)1 |
0.90 (0.77, 1.05) |
|
|
Overall response rate (per investigator) |
||
|
ORR, % (95% CI)4,5 |
20.1 (16.3, 24.4) |
5.1 (3.2, 7.8) |
|
Complete response, n (%) |
12 (3.1) |
0 |
|
Partial response, n (%) |
67 (17.0) |
20 (5.1) |
|
Duration of response (per investigator) |
||
|
Median DoR in months (95% CI) |
22.3 (13.7, NR) |
18.4 (6.5, 26.0) |
|
% with duration ≥6 months |
82.3 |
78.9 |
|
% with duration ≥12 months |
65.8 |
63.2 |
Source: IMJUDO Prescribing Information
1 HR (IMJUDO and durvalumab versus sorafenib) based on the stratified Cox proportional hazard model.
2 Based on a stratified log-rank test.
3 Based on a Lan-DeMets alpha spending function with O'Brien Fleming type boundary and the actual number of events observed, the boundary for declaring statistical significance for IMJUDO and durvalumab versus sorafenib was 0.0398 (Lan and DeMets 1983).
4 Confirmed complete response or partial response.
5 Based on Clopper-Pearson method.
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: CI, confidence interval; DoR, duration of response; HR, hazard ratio; n, number of patients with event; N, number of patients in treatment arm; NR, not reached; ORR, objective response rate; PFS, progression free survival
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: IMJUDO plus durvalumab worked similarly in males and females.
- Race: The majority of patients were Asian or White. IMJUDO plus durvalumab worked similarly in these populations. Due to the small number of patients in other racial subgroups, differences in response among racial groups could not be determined.
- Age: IMJUDO plus durvalumab worked similarly in patients younger and older than 65 years of age
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Observations of efficacy based on subgroups of the population studied are provided in Table 3.
Table 3. Overall Survival Subgroup Analysis (Efficacy Population)
|
Subgroup |
IMJUDO + |
Sorafenib |
HR |
95% CI |
|---|---|---|---|---|
|
All patients |
262/393 (66.7) |
293/389 (75.3) |
0.78 |
0.66, 0.92 |
|
Sex |
||||
|
Male |
211/327 (64.5) |
255/337 (75.7) |
0.73 |
0.61, 0.88 |
|
Female |
51/66 (77.3) |
38/52 (73.1) |
1.02 |
0.67, 1.56 |
|
Age at randomization, years |
||||
|
<65 |
134/195 (68.7) |
146/195 (74.9) |
0.82 |
0.65, 1.04 |
|
≥65 |
128/198 (64.6) |
147/194 (75.8) |
0.73 |
0.58, 0.93 |
|
Race |
||||
|
White |
127/182 (69.8) |
135/179 (75.4) |
0.85 |
0.67, 1.08 |
|
Asian |
128/195 (65.6) |
144/189 (76.2) |
0.73 |
0.57, 0.92 |
|
Others |
7/16 (43.8) |
14/21 (66.7) |
0.61 |
0.23, 1.48 |
Source: Adapted from FDA Review
Efficacy population includes patients treated with IMJUDO + durvalumab and patients treated with sorafenib
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: CI, confidence interval; HR, hazard ratio; n, number of patients meeting criteria; N, number of patients in treatment arm; Ns, number of patients in subgroup
What are the possible side effects?
The most common side effects (≥20%) in patients treated with IMJUDO are rash, diarrhea (loose stools) or more frequent bowel movements than usual, extreme tiredness, itching of the skin, muscle pain, and abdominal pain.
IMJUDO may cause serious side effects including inflammation of the lung (pneumonitis), inflammation of the liver (hepatitis), inflammation of the intestines (colitis), hormone glands injury (pituitary, thyroid, adrenal, pancreas), inflammation of the kidney (nephritis), and may harm an unborn baby.
What are the possible side effects (results of trials used to assess safety)?
The possible side effects occurring in ≥10% of patients in the study where IMJUDO was used in combination with durvalumab are detailed in Table 4.
Table 4. Adverse Reactions Occurring in ≥10% Patients
|
Adverse Reaction |
IMJUDO + Durvalumab |
Sorafenib |
||
|---|---|---|---|---|
|
All Grades |
Grade 3 to 4 |
All Grades |
Grade 3 to 4 |
|
|
Gastrointestinal disorders |
||||
|
Diarrhea1 |
27 |
6 |
45 |
4.3 |
|
Abdominal pain1 |
20 |
1.8 |
24 |
4 |
|
Nausea |
12 |
0 |
14 |
0 |
|
Skin and subcutaneous tissue disorders |
||||
|
Rash1 |
32 |
2.8 |
57 |
12 |
|
Pruritus |
23 |
0 |
6 |
0.3 |
|
Metabolism and nutrition disorders |
||||
|
Decreased appetite |
17 |
1.3 |
18 |
0.8 |
|
General disorders and administration site conditions |
||||
|
Fatigue1 |
26 |
3.9 |
30 |
6 |
|
Pyrexia1 |
13 |
0.3 |
9 |
0.3 |
|
Psychiatric disorders |
||||
|
Insomnia |
10 |
0.3 |
4.3 |
0 |
|
Endocrine disorders |
||||
|
Hypothyroidism1 |
14 |
0 |
6 |
0 |
|
Musculoskeletal and connective tissue disorders |
||||
|
Musculoskeletal pain1 |
22 |
2.6 |
17 |
0.8 |
Source: IMJUDO Prescribing Information
1 Represents a composite of multiple related terms.
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: N, number of patients
Were there any differences in side effects of the clinical trials among sex, race, and age?
- Sex: The occurrence of side effects was similar in males and females. The occurrence of more serious side effects (grade ≥3) in patients treated with IMJUDO plus durvalumab was higher in females.
- Race: Most patients were Asian or White, the occurrence of side effects was similar across these racial groups. The occurrence of more serious side effects (grade ≥3) in patients treated with IMJUDO plus durvalumab was higher in White than Asian patients. Due to the small number of patients in other racial groups, differences in side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age. The occurrence of more serious side effects (grade ≥3) in patients treated with IMJUDO plus durvalumab was higher in patients older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 5, Table 6, and Table 7 summarize adverse reactions that were reported during the trial.
Table 5. Treatment Emergent Adverse Events by Sex – IMJUDO + Durvalumab Safety Population
|
Parameter |
Male |
Female |
|---|---|---|
|
Patients with TEAEs |
314 (97.2) |
64 (98.5) |
|
Patients with TEAEs grade ≥3 |
172 (53.3) |
39 (60.0) |
|
Patients with serious TEAEs |
131 (40.6) |
26 (40.0) |
Source: Adapted from FDA Review
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: n, number of patients with criteria; N, number of patients; TEAE, treatment-emergent adverse event
Table 6. Treatment Emergent Adverse Events by Race – IMJUDO + Durvalumab Safety Population
|
Parameter |
White |
Asian |
All Other Races |
|---|---|---|---|
|
Patients with TEAEs |
176 (99.4) |
186 (95.4) |
15 (100.0) |
|
Patients with TEAEs grade ≥3 |
107 (60.5) |
96 (49.2) |
8 (53.3) |
|
Patients with serious TEAEs |
75 (42.4) |
77 (39.5) |
5 (33.3) |
Source: Adapted from FDA Review
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: n, number of patients with criteria; N, number of patients; TEAE, treatment-emergent adverse event
Table 7. Treatment Emergent Adverse Events by Age – IMJUDO + Durvalumab Safety Population
|
Parameter |
<65 Years |
≥65 Years |
|---|---|---|
|
Patients with TEAEs |
186 (96.4) |
192 (98.5) |
|
Patients with TEAEs grade ≥3 |
98 (50.8) |
113 (57.9) |
|
Patients with serious TEAEs |
75 (38.9) |
82 (42.1) |
Source: Adapted from FDA Review
IMJUDO + Durvalumab: IMJUDO given as a single dose with durvalumab on Day 1 then durvalumab every 4 weeks
Abbreviations: n, number of patients with criteria; N, number of patients; TEAE, treatment-emergent adverse event
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.