Drug Trials Snapshots: EMRELIS
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the EMRELIS Prescribing Information for all the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
EMRELIS (telisotuzumab vedotin-tllv)
EM-rell-is
AbbVie Inc.
Approval date: May 14, 2025
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
EMRELIS is a prescription drug used for the treatment of adult patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) with high c Met protein overexpression, who have received a prior systemic anti-cancer therapy.
How is this drug used?
EMRELIS is given into a vein (intravenous infusion) by a healthcare professional once every two weeks.
Who participated in the clinical trials?
FDA granted accelerated approval to EMRELIS based predominantly on evidence from one clinical trial (LUMINOSITY/NCT03539536) of 168 patients with non-squamous, epidermal growth factor receptor (EGFR) wild-type NSCLC with c Met protein overexpression who had received prior systemic therapy, including 19 patients from the United States. The trial was conducted at 119 sites across 23 countries in North America, Europe, Asia, the Middle East, and Oceania. There were 84 patients with non-squamous, EGFR wild-type NSCLC with high c-Met protein overexpression who had received prior systemic therapy.
How were the trials designed?
The benefits and side effects of EMRELIS were evaluated in one clinical trial of 168 patients with non-squamous, EGFR wild-type NSCLC with high c-Met protein overexpression who had received one to three prior systemic treatments.
How were the trials designed?
EMRELIS was evaluated in LUMINOSITY, a multicenter, open-label, single-arm, multi-cohort clinical trial. Patients received EMRELIS at 1.9 mg/kg as an infusion into the vein every two weeks until disease progression or unacceptable toxicity. The major efficacy outcome measure was overall response rate (ORR) according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 as assessed by a blinded independent central review (BICR). An additional efficacy outcome measure was duration of response (DOR) by BICR.
The efficacy population included 84 patients with non-squamous, EGFR wild-type NSCLC with high c-Met protein overexpression who had received prior systemic therapy.
The safety population reflects exposure to EMRELIS in 168 patients with locally advanced or metastatic EGFR wild-type non-squamous NSCLC with c-Met protein overexpression who received EMRELIS as a single agent.
DEMOGRAPHICS SNAPSHOT
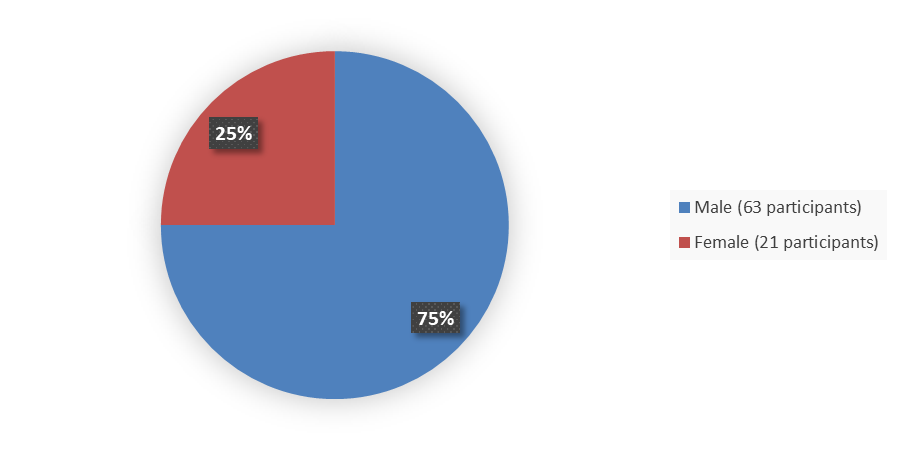
Figure 1 summarizes the percentage of patients by sex enrolled in the clinical trial used to evaluate the efficacy of EMRELIS.
Figure 1. Baseline Demographics by Sex, Efficacy Population
Source: Adapted from FDA Review
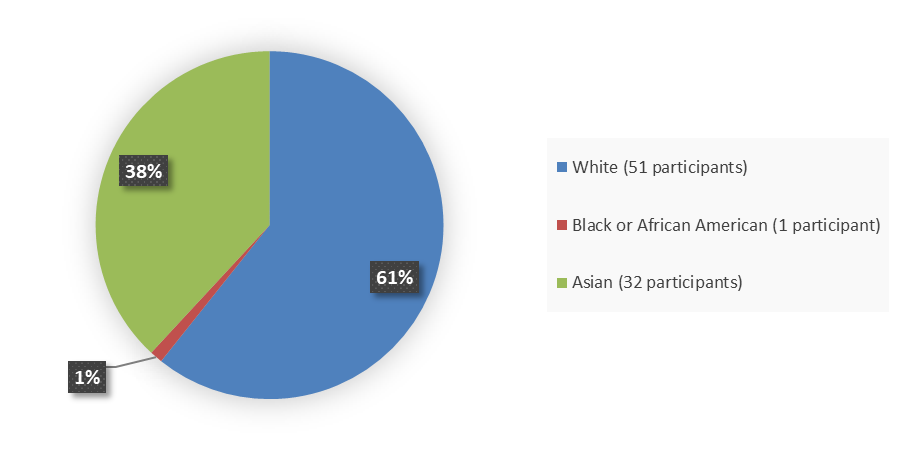
Figure 2 summarizes the percentage of patients by race enrolled in the clinical trial used to evaluate the efficacy of EMRELIS.
Figure 2. Baseline Demographics by Race, Efficacy Population
Source: Adapted from FDA Review
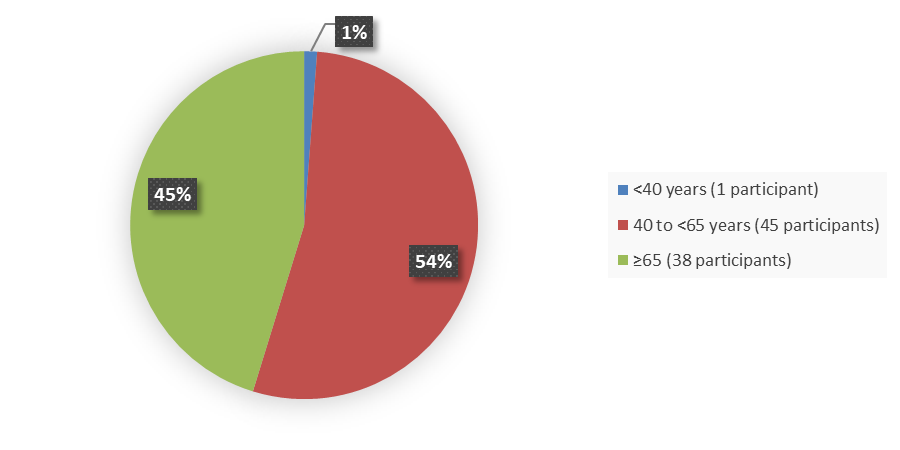
Figure 3 summarizes the percentage of patients by age group enrolled in the clinical trial used to evaluate the efficacy of EMRELIS.
Figure 3. Baseline Demographics by Age Group, Efficacy Population
Source: Adapted from FDA Review
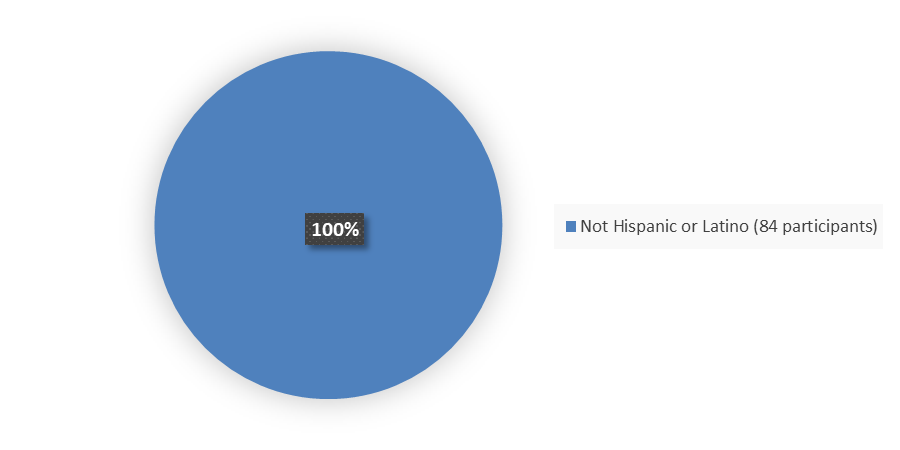
Figure 4 summarizes the percentage of patients by ethnicity enrolled in the clinical trial used to evaluate the efficacy of EMRELIS.
Figure 4. Baseline Demographics by Ethnicity, Efficacy Population
Source: Adapted from FDA Review
Who participated in the trials?
Table 1 shows the baseline demographics for the efficacy and safety populations.
Table 1. Baseline Demographics of LUMINOSITY, Efficacy and Safety Populations
| Demographic | Efficacy Population N=84 n (%) | Safety Population N=168 n (%) |
|---|---|---|
| Sex | ||
| Male | 63 (75.0) | 117 (69.6) |
| Female | 21 (25.0) | 51 (30.4) |
| Age group, years | ||
| <40 | 1 (1.2) | 2 (1.2) |
| 40 to <65 | 45 (53.6) | 82 (48.8) |
| ≥65 | 38 (45.2) | 84 (50.0) |
| Race | ||
| White | 51 (60.7) | 110 (65.5) |
| Black or African American | 1 (1.2) | 3 (1.8) |
| Asian | 32 (38.1) | 55 (32.7) |
| Ethnicity | ||
| Hispanic or Latino | 0 | 1 (0.6) |
| Non-Hispanic or Latino | 84 (100) | 167 (99.4) |
Source: Adapted from FDA Review
What are the benefits of this drug?
The LUMINOSITY trial measured the percentage of patients whose tumors completely or partially shrank after treatment (ORR). In the trial, 35% of 84 patients taking EMRELIS experienced complete or partial shrinkage of their tumors, which lasted a median of approximately seven months.
EMRELIS was approved under FDA’s accelerated approval program. This program lets patients take a promising new drug while the company continues clinical trials to confirm that the drug works well.
What are the benefits of this drug (results of trials used to assess efficacy)?
Table 2 shows the efficacy results in LUMINOSITY for patients with EGFR wild-type non-squamous NSCLC with high c-Met protein overexpression.
Table 2. Efficacy Results, Efficacy Population
| Efficacy Parameter | EMRELIS |
|---|---|
| Confirmed ORR, % (95% CI) | 35 (24, 46) |
| Complete response, % | 0 |
| Partial response, % | 35 |
| Duration of response | N=29 |
| Median, months, (95% CI) | 7.2 (4.2, 12) |
| DOR ≥6 monthsa, % | 59 |
| DOR ≥12 monthsa, % | 21 |
Source: Adapted from FDA Review
a Based on observed duration of response in 29 responders
Abbreviations: CI, confidence interval; DOR, duration of response; ORR, overall response rate
Were there any differences in how well the drug worked in clinical trials among sex, race, and age?
- Sex: EMRELIS worked consistently in males and females.
- Race: There was only one patient in the Black or African American subgroup limiting conclusions for this subgroup; otherwise, ORR results were consistent across subgroups studied.
- Age: EMRELIS worked consistently in patients younger than 65 years of age and 65 years of age and above.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age
Table 3 summarizes efficacy results by sex, race, and age group based on the overall response rate.
Table 3. Efficacy Results by Sex, Race, and Age, Efficacy Population
| Demographic | Efficacy Population | Responder | ORR (95% CI) |
|---|---|---|---|
| All patients | 84 | 29 | 35 (24, 46)
|
| Sex |
|
|
|
| Male | 63 | 21 | 33 (22, 46) |
| Female | 21 | 8 | 38 (18, 62) |
| Race |
|
|
|
| White | 51 | 15 | 29 (17, 44) |
| Black or African American | 1 | 0 | 0 (0, 0.98) |
| Asian | 32 | 14 | 44 (26, 62) |
| Age, years |
|
|
|
| <65 | 46 | 14 | 30 (18, 46) |
| ≥65 | 38 | 15 | 39 (24, 57) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; ORR, overall response rate
What are the possible side effects?
The most common side effects of EMRELIS include nerve problems in your hands or feet (peripheral neuropathy), feeling tired (fatigue), decreased appetite, and swelling in the feet, ankles, legs, or hands (peripheral edema).
EMRELIS can cause serious side effects, including peripheral neuropathy, lung problems, eye problems, infusion-related reactions, or harm to an unborn baby.
What are the possible side effects (results of trials used to assess safety)?
Table 4 summarizes the common adverse reactions that occurred in the clinical trial.
Table 4. Adverse Reactions (≥10%) in Patients With EGFR Wild-Type Non-Squamous NSCLC With c-Met Protein Overexpression in LUMINOSITY, Safety Population
| Adverse Reaction | EMRELIS, N=168 | |
|---|---|---|
| All Grades1 % | Grade 3 or 41 % | |
| Nervous system disorders | ||
| Peripheral neuropathy2 | 51 | 11 |
| General disorders and administration site conditions | ||
| Fatigue3 | 29 | 3.6 |
| Peripheral edema2 | 22 | 1.8 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 22 | 0.6 |
| Gastrointestinal disorders | ||
| Nausea | 15 | 0 |
| Constipation | 14 | 0.6 |
| Vomiting | 10 | 0.6 |
| Eye disorders | ||
| Blurred vision4 | 15 | 1.2 |
| Keratitis5 | 11 | 0.6 |
| Infections and infestations | ||
| Pneumonia2 | 13 | 6 |
| Respiratory, thoracic and mediastinal disorders | ||
| ILD/pneumonitis2 | 10 | 3.6 |
Source: Source: Adapted from FDA Review
1 Events were graded using NCI CTCAE version 4.03
2 Grouped term
3 Includes fatigue and asthenia
4 Includes vision blurred, visual acuity reduced, and visual impairment
5 Includes corneal cyst, corneal disorder, corneal erosion, corneal edema, corneal opacity, keratitis, keratitis interstitial, and punctate keratitis
Abbreviations: EGFR, epidermal growth factor receptor; ILD, interstitial lung disease; NCI CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; NSCLC, non-small cell lung cancer
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The occurrence of side effects was similar in White and Asian. There were only three patients in the Black or African American subgroup limiting conclusions for this subgroup.
- Age: The occurrence of side effects was similar in patients younger than 65 years of age and 65 years of age and above.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.