Drug Trials Snapshot: PYRUKYND
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the PYRUKYND Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
PYRUKYND (mitapivat)
(pye roo' kind)
AGIOS PHARMACEUTICALS INC
Approval date: February 17, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
PYRUKYND is a drug used to treat hemolytic anemia in adults with pyruvate kinase (PK) deficiency.
Pyruvate kinase deficiency is a rare inherited genetic disorder in which red blood cells are destroyed prematurely leading to anemia (low red blood cell counts). Some patients may have severe symptoms and need regular blood transfusions.
How is this drug used?
PYRUKYND is a tablet that is taken by mouth twice daily.
What are the benefits of this drug?
In patients who did not require regular blood transfusions, PYRUKYND was better than placebo in increasing hemoglobin (a measure of amount of red blood cells that are not destroyed). In the trial, 40% of patients treated with PYRUKYND achieved a pre-determined increase in hemoglobin in comparison to none of the patients who received placebo.
In patients who received regular blood transfusions, PYRUKYND decreased the need for red blood cell transfusions. In the trial, 33% of patients who received PYRUKYND achieved a pre-determined reduction in red blood cell red transfusion, including 22% of patients who did not require any transfusions.
Who participated in the clinical trials?
The FDA approved PYRUKYND based on evidence from two clinical trials . Trial 1 (NCT03548220) of 80 adults with PK deficiency who did not receive regular blood transfusions and Trial 2 (NCT03559699) of 27 adults with PK deficiency who received regular blood transfusions.
Trial 1 was conducted at 36 sites in the following countries: Brazil, Canada, Denmark, France, Germany, Italy, Japan, Republic of Korea, Netherlands, Spain, Switzerland, Turkey, United Kingdom and United States.
Trial 2 was conducted at 17 sites in the following countries: Canada, Denmark, France, Ireland, Italy, Netherlands, Thailand, United Kingdom and United States.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results from the trial in patients who did not receive regular blood transfusions. The results are based on the proportion of patients who achieved hemoglobin response defined as 1.5 g/dL or greater increase in hemoglobin concentration from the beginning of the study that was sustained at two or more scheduled assessments during the last 12 weeks of treatment.
Table 1. Efficacy Results in Patients with PK Deficiency Who Were Not Regularly Transfused
|
Endpoint |
PYRUKYND |
Placebo |
Difference |
p-value2 |
|---|---|---|---|---|
|
Hb Response1 |
16 (40%) |
0 |
39 (24, 55) |
<0.0001 |
CI: confidence interval, Hb: hemoglobin,
- For Hb response, the difference is adjusted for randomization stratification factors, which included average screening Hb (<8.5, ≥8.5 g/dL) and PKLR gene variant category (missense/missense, missense/non-missense).
- The two-sided p-value is based on the Mantel-Haenszel stratum weighted method adjusting for the randomization stratification factors.
Adapted from PYRUKYND Prescribing Information.
The table below summarizes efficacy results from the trial in patients who received regular blood transfusions. The results are based on the proportion of patients who achieved reduction on transfusion burden defined at least a 33% reduction in the number of red blood cell units transfused during the last 24 weeks of treatment compared with the patient’s historical transfusion burden (standardized to 24 weeks).
Table 2. Efficacy Results in Patients with PK Deficiency Who Were Regularly Transfused
|
Endpoints |
PYRUKYND |
|---|---|
|
Patients with Transfusion Reduction Response |
|
|
Patients who were Transfusion Free |
|
CI: confidence interval, CI is based on Clopper-Pearson method.
Adapted from PYRUKYND Prescribing Information.
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: PYRUKYND worked similarly in men and women.
- Race: PYRUKYND worked similarly in all tested race groups.
- Age: PYRUKYND worked similarly in all tested age groups. There were not enough patients aged 65 years and older to determine whether there was any difference between older and younger patients.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The tables below summarize efficacy results by sex, race and age.
Due to the small sample size, these exploratory analyses should be interpreted with caution.
Table 3. Subgroup Analyses of Hemoglobin Response by Sex, Race and Age in Patients with PK Deficiency Who Were Not Regularly Transfused
|
Subgroup |
PYRUKYND |
Placebo |
|---|---|---|
|
Sex |
||
|
Male |
4/16 25 (7, 52) |
0/16 0 (0, 21) |
|
Female |
12/24 50 (29, 71) |
0/24 0 (0,14) |
|
Race |
||
|
White |
13/28 46 (28, 66) |
0/32 0 (0,11) |
|
Asian |
1/5 20 (1, 72) |
0/3 0 (0, 71) |
|
Not reported |
1/6 17 (0, 64) |
0/4 0 (0, 60) |
|
Other |
1/1 100 (3, 100) |
0/1 0 (0, 98) |
|
Age (years) |
||
|
< 35 |
9/22 41 (21, 64) |
0/20 0 (0, 17) |
|
≥ 35 |
7/18 39 (17, 64) |
0/20 0 (0, 17) |
Table 4. Subgroup Analyses of Transfusion Burden Reduction by Sex, Race and Age in Patients with PK Deficiency Who Were Regularly Transfused
|
Subgroup |
PYRUKYND |
|---|---|
|
Sex |
|
|
Male |
2/7 29 (4, 71) |
|
Female |
7/20 35 (15, 59) |
|
Race |
|
|
White |
6/20 30 (12, 54) |
|
Asian |
1/3 33 (1, 91) |
|
Not reported
|
2/4 50 (7, 93) |
|
Age (years) |
|
|
< 35 |
4/13 31 (9, 61) |
|
≥ 35 |
5/14 36 (13, 65) |
What are the possible side effects?
PYRUKYND may cause serious side effects such as rapid breakdown of red blood cells (acute hemolysis) after suddenly interrupting or stopping treatment with PYRUKYND. Patients should avoid suddenly stopping or pausing PYRUKYND and follow their health care provider’s instructions for discontinuing treatment.
The most common side effects of PYRUKYND are decreases in estrone and estradiol (types of the estrogen hormone) in men, increased urate (a type of salt in the body), back pain, and joint stiffness. The effects of PYRUKYND on estrone and estradiol could not be reliably assessed in women because of normal changes in these hormones during the menstrual cycle and use of hormonal contraception.
What are the possible side effects (results of trials used to assess safety)?
The table below summarizes adverse reactions that occurred in the clinical trials.
Table 5. Adverse Reactions (≥ 5%) in Patients Receiving PYRUKYND
|
Adverse Reactions |
PYRUKYND |
Placebo |
||
|---|---|---|---|---|
|
All Grades |
Grade ≥3 |
All Grades |
Grade ≥3 |
|
|
Back paina |
15% |
0 |
8% |
0 |
|
Arthralgiab |
10% |
0 |
5% |
0 |
|
Hypertriglyceridemiac |
8% |
5% |
3% |
0 |
|
Gastroenteritis |
8% |
3% |
0 |
0 |
|
Hot flushd |
8% |
0 |
0 |
0 |
|
Oropharyngeal pain |
8% |
0 |
5% |
0 |
|
Hypertension |
5% |
5% |
0 |
0 |
|
Arrhythmiae |
5% |
0 |
0 |
0 |
|
Breast discomfort |
5% |
0 |
0 |
0 |
|
Constipation |
5% |
0 |
0 |
0 |
|
Dry mouthf |
5% |
0 |
0 |
|
|
Paresthesia |
5% |
0 |
0 |
0 |
Grades: Per the CTCAE definition.
Grouped Term Definitions
a Includes back pain, sciatica, and flank pain.
b Includes arthralgia and joint swelling.
c Includes hypertriglyceridemia and blood triglycerides increased.
d Includes hot flush and flushing.
e Includes arrhythmia, tachycardia, heart rate increased and atrial fibrillation.
f Includes dry mouth and dry lip.
Laboratory abnormalities of PYRUKYND included increased urate (15%).
The table below summarizes the laboratory abnormalities in reproductive hormones in men.
Table 6. Laboratory Abnormalities in Reproductive Hormones in Men Receiving PYRUKYND
|
Parameter |
PYRUKYND |
Placebo |
|---|---|---|
|
Reproductive hormone analysesa |
||
|
Estrone decreased (males) |
9 (56) |
0 |
a Decreases in estrone and estradiol to below the lower limit of the reference range and increases in testosterone to above the upper limit of the reference range where baseline was within normal limits.
Adapted from PYRUKYND Prescribing Information.
Were there any differences in side effects among sex, race, and age?
- Sex: The occurrence of side effects was similar in men and women.
- Race: The occurrence of side effects was similar in all tested groups.
- Age: The occurrence of side effects was similar in age groups under 65 years. There were not enough patients aged 65 years and older to determine whether there was any difference between older and younger patients.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize adverse reactions by subgroups.
Due to the small sample size, these exploratory analyses should be interpreted with caution.
Table 7. Adverse Reactions by Sex Subgroups
|
|
PYRUKYND (n=40) |
Placebo (n=39) |
||
|---|---|---|---|---|
|
Male |
Female |
Male |
Female |
|
|
All TEAEs |
14 (87.5) |
21 (87.5) |
14 (93.3) |
21 (87.5) |
|
Serious TEAEs |
2 (12.5) |
2 (8.3) |
1 (6.7) |
1 (4.2) |
Table 8. Adverse Reactions by Race Subgroups
|
|
PYRUKYND (n=40) |
Placebo (n=39) |
||
|---|---|---|---|---|
|
White |
Other |
White |
Other |
|
|
All TEAEs |
24 (85.7) |
11 (91.7) |
28 (87.5) |
7 (100) |
|
Serious TEAEs |
2 (7.1) |
2 (16.7) |
2 (6.3) |
0 |
Table 9. Adverse Reactions by Age Subgroups
|
|
PYRUKYND (n=40) |
Placebo (n=39) |
||
|---|---|---|---|---|
|
<35 years |
≥ 35 years |
<35 years |
≥ 35 years |
|
|
All TEAEs |
20 (90.9) |
15 (83.3) |
18 (94.7) |
17 (85.0) |
|
Serious TEAEs |
1 (4.5) |
3 (16.7) |
1 (5.3) |
1 (5.0) |
TEAE=treatment -emergent adverse events; SAE=serious adverse events
Adapted from Clinical Trial Data
DEMOGRAPHICS SNAPSHOT
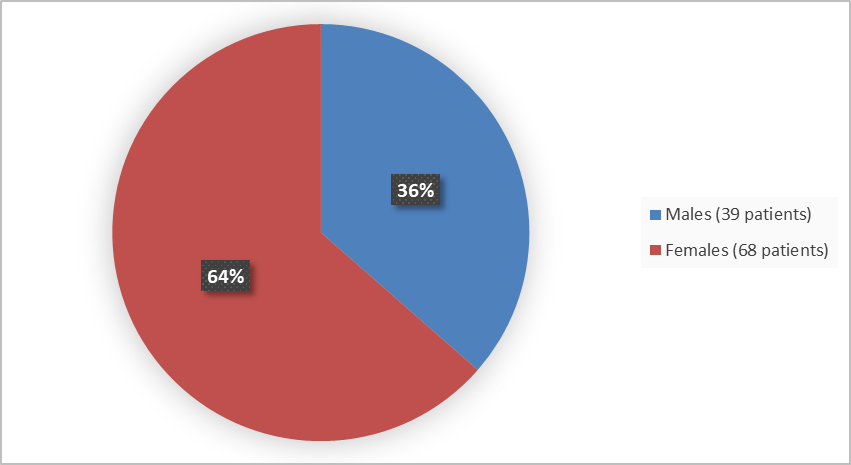
Figure 1 summarizes how many males and females were in the clinical trials.
Figure 1. Baseline Demographics by Sex
Figure 2 summarizes the percentage of patients by race in the clinical trials.
Figure 2. Baseline Demographics by Race
Table 10. Demographics by Race
|
Race |
Number of Patients |
Percentage |
|---|---|---|
|
White |
80 |
75 |
|
Asian |
11 |
10 |
|
Other |
16 |
15 |
|
Not reported |
14 |
13 |
Adapted from FDA Review
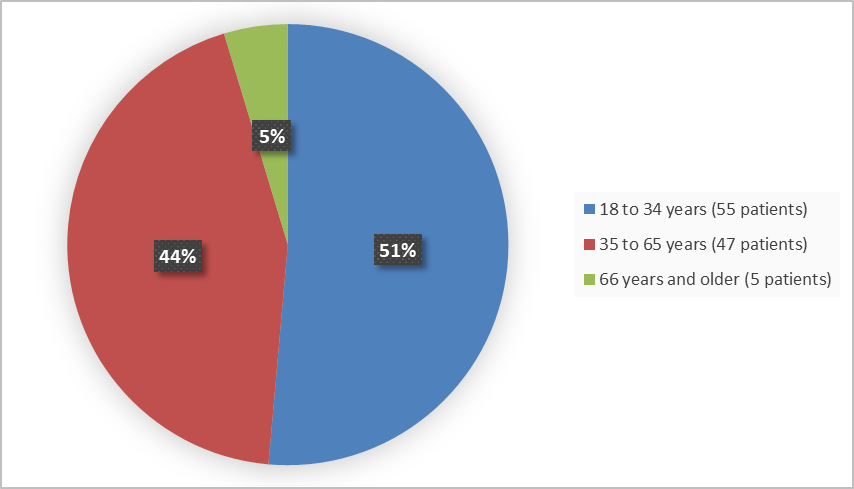
Figure 3 summarizes the percentage of patients by age group in the clinical trials.
Figure 3. Baseline Demographics by Age
Who participated in the trials?
The table below summarizes demographics of patients that participated in both trials.
Table 11. Trial Demographics (Trials 1 and 2 combined)
|
Demographic Parameter |
PYRUKYND |
Placebo |
Total |
|---|---|---|---|
|
Sex |
|||
|
Women |
44 (66) |
24 (60) |
68 (64) |
|
Men |
23 (34) |
16 (40) |
39 (36) |
|
Race |
|||
|
White |
48 (72) |
32 (80) |
80 (75) |
|
Asian |
8 (12) |
3 (8) |
11 (10) |
|
Not reported |
10 (15) |
4 (10) |
14 (13) |
|
Other |
1 (2) |
1 (3) |
2 (2) |
|
Age |
|||
|
Mean (SD) |
36.2 (14.6) |
37.2 (15.9) |
36.6 (15.0) |
|
Median |
34 |
35.5 |
34 |
|
Range |
18-70 |
19-78 |
|
|
Age Group |
|||
|
<35 |
35 (52) |
20 (50) |
55 (51) |
|
≥35 to ≤65 |
29 (43) |
18 (45) |
47 (44) |
|
>65 |
3 (5) |
2 (5) |
5 (5) |
|
Ethnicity |
|||
|
Hispanic or Latino |
2 (3) |
1 (3) |
3 (3) |
|
Not Hispanic or Latino |
48 (72) |
34 (85) |
82 (77) |
|
Missing |
17 (25) |
5 (13) |
22 (21) |
|
Region |
|||
|
Asia |
7 (10) |
3 (8) |
10 (9) |
|
Western Europe |
40 (60) |
20 (50) |
60 (56) |
|
North America |
19 (28) |
16 (40) |
35 (33) |
|
US only |
17 (25) |
14 (35) |
31 (29) |
|
Rest of the world |
1 (2) |
1 (3) |
2 (3) |
Adapted from FDA Review
How were the trials designed?
PYRUKYND was evaluated in two clinical trials of 107 patients with pyruvate kinase (PK) deficiency. Trial 1 was conducted in patients who were not regularly transfused and Trial 2 in patients who received regular blood transfusions.
In Trial 1, patients were randomly assigned to receive either PYRUKYND or a matched placebo tablet for an average duration of about 24 weeks. Neither the patients nor the healthcare providers knew which treatment was being given during the trial.
In Trial 2, all patients received PYRUKYND for an average duration of about 40 weeks.
In Trial 1, the benefit of PYRUKYND was assessed by the percentage of patients who achieved a pre-determined increase in hemoglobin.
In Trial 2, the benefit of PYRUKYND was assessed by the percentage of patients who had a pre-determined reduction in red blood cell red transfusions.
How were the trials designed?
The safety and efficacy evaluation of PYRUKYND for the treatment of hemolytic anemia in adults with PK deficiency was based on two clinical trials.
Trial 1 was a randomized, double-blind, placebo-controlled trial of adults with PK deficiency who did not require regular blood transfusions (no more than 4 transfusion episodes per year).
Trial 2 was a single-arm trial of adults with PK deficiency who received regular blood transfusions (at least 6 transfusion episodes per year).
In Trials 1 and 2, patients received PYRUKYND up to 50 mg orally twice daily after an initial dose titration (adjustment) period.
The major efficacy outcome in patients who did not receive regular blood transfusion (Trial 1) was hemoglobin response defined as a 1.5 g/dL or greater increase in hemoglobin concentration from the beginning of the study that was sustained at two or more scheduled assessments.
In Trial 2, efficacy was based on reduction in transfusion burden, defined as at least a 33% reduction in the number of red blood cell units transfused during the last 24 weeks of treatment compared with the historical transfusion burden on the individual participant (standardized to 24 weeks).
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.