Drug Trial Snapshots: AMVUTTRA
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the key clinical trials that supported the original FDA approval of this drug, and whether there were differences among sex, race, age, and ethnic groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your healthcare provider about the benefits and risks of a drug.
Some of the information in this Snapshot is for presentation purposes and does not represent the approved conditions of use of this drug. Refer to the AMVUTTRA Prescribing Information for all of the approved conditions of use of this drug (e.g., indication(s), population(s), dosing regimen(s), safety information).
Snapshots are limited to the information available at the time of the original approval of the drug and do not provide information on who participated in clinical trials that supported later approvals for additional uses of the drug (if applicable).
AMVUTTRA (vutrisiran)
(am vue´ tra)
Alnylam Pharmaceuticals, Inc.
Approval date: June 13, 2022
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
AMVUTTRA is a drug for the treatment of nerve damage in adult patients with hereditary transthyretin-mediated amyloidosis. Transthyretin-mediated amyloidosis is the buildup of abnormal deposits of a substance called amyloid in the body's organs and tissues. Amyloid disrupts the function of organs and tissues.
How is this drug used?
AMVUTTRA is injected under the skin by a healthcare professional once every three months.
Who participated in the clinical trials?
The FDA approved AMVUTTRA based on evidence from one clinical trial (Trial 1/NCT03759379) in which 122 patients with hereditary transthyretin-mediated amyloidosis received AMVUTTRA.
The trial was conducted at 57 sites in 22 countries and of the 122 patients, 26 patients were from trial sites in the United States.
How were the trials designed?
The benefits and side effects of AMVUTTRA were evaluated in one clinical trial. The trial enrolled patients who had hereditary transthyretin-mediated amyloidosis (Trial 1/NCT03759379). Patients received AMVUTTRA subcutaneously once every three months.
Healthcare providers rated the change in the signs and symptoms of neuropathy (nerve damage) from baseline to Month 9 using a numerical scale. The scores for the patients receiving AMVUTTRA were compared to the scores for patients receiving placebo in a previously conducted study.
How were the trials designed?
The efficacy and safety of AMVUTTRA were established in one clinical trial. The trial evaluated AMVUTTRA for the treatment of polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. The primary efficacy endpoint was the change from baseline to Month 18 in the mNIS+7.
The mNIS+7 is an objective assessment of neuropathy. It measures deficits in cranial nerve function, muscle strength, reflexes, and sensation. Health care providers rate severity on a maximum possible score of 304 points. Higher scores represent greater severity of disease. The clinical meaningfulness of the objective findings on the mNIS+7 were supported by the Norfolk QoL-DN scale that evaluated the functional impact of the observed changes on the mNIS+7. Additional measurements were gait speed, as measured by the 10-meter walk test, and modified body mass index.
DEMOGRAPHICS SNAPSHOT
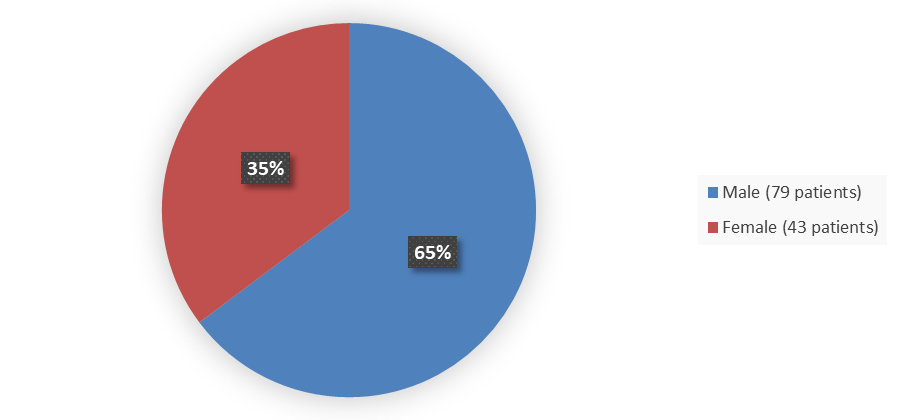
Figure 1 summarizes how many male and female patients were in the clinical trial used to evaluate efficacy and safety.
Figure 1. Baseline Demographics by Sex
Source: Adapted from FDA Review
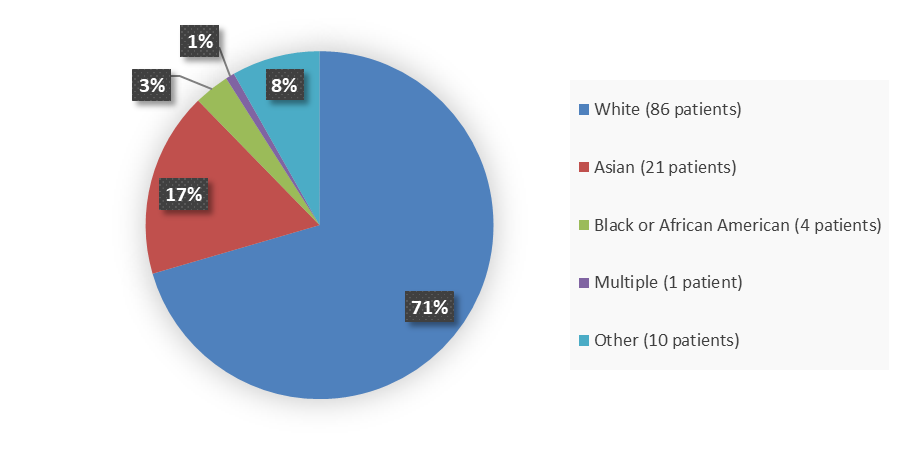
Figure 2 summarizes the percentage of patients by race in the clinical trial used to evaluate efficacy and safety.
Figure 2. Baseline Demographics by Race
Source: Adapted from FDA Review
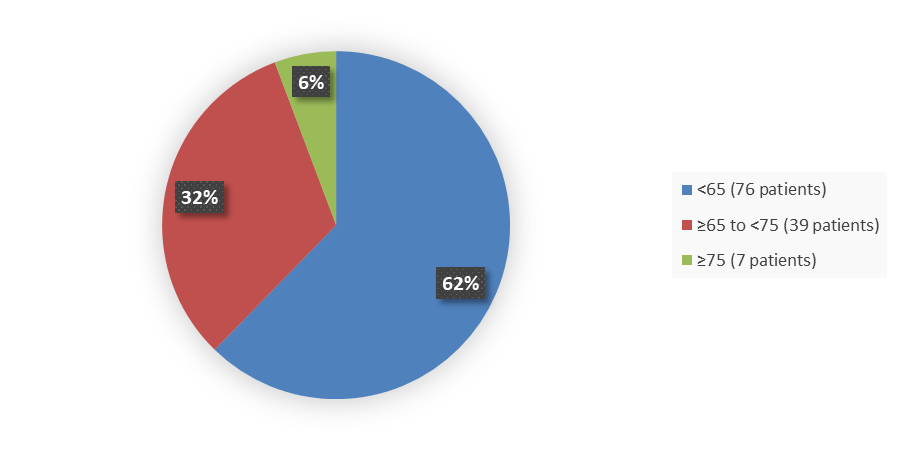
Figure 3 summarizes the percentage of patients by age enrolled in the clinical trial used to evaluate efficacy and safety.
Figure 3. Baseline Demographics by Age
Source: Adapted from FDA Review
Who participated in the trials?
Table 5 summarizes demographics of all patients in the clinical trial.
Table 5. Demographic Characteristics for Trial 1
| Demographic | AMVUTTRA N=122 |
External Placebo N=77 |
|---|---|---|
| Sex, n (%) | ||
| Female | 43 (35.2) | 19 (24.7) |
| Male | 79 (64.8) | 58 (75.3) |
| Age, years | ||
| Mean (SD) | 57.80 (13.19) | 62.17 (10.76) |
| Median (min, max) | 60.00 (26.0, 85.0) | 63.00 (34.0, 80.0) |
| Age groups, years, n (%) | ||
| <65 | 76 (62.3) | 44 (57.1) |
| 65 to <75 | 39 (32.0) | 24 (31.2) |
| ≥75 | 7 (5.7) | 9 (11.7) |
| Race, n (%) | ||
| Asian | 21 (17.2) | 25 (32.5) |
| Black or African American | 4 (3.3) | 1 (1.3) |
| Multiple | 1 (0.8) | 0 (0.0) |
| Other | 10 (8.2) | 0 (0.0) |
| Unknown | 0 (0.0) | 1 (1.3) |
| White | 86 (70.5) | 50 (64.9) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 12 (9.8) | 11 (14.3) |
| Not Hispanic or Latino | 109 (89.3) | 65 (84.4) |
| Unknown | 1 (0.8) | 1 (1.3) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
What are the benefits of this drug?
Compared to patients receiving placebo, patients who received AMVUTTRA had fewer symptoms of nerve damage as well as better muscle strength.
What are the benefits of this drug?
Table 1 summarizes efficacy results for the evaluated patients for Trial 1. The main trial endpoints were the change from baseline to Month 9 on the Norfolk Quality of Life Diabetic Neuropathy (Norfolk QoL-DN) total score and the modified Neuropathy Impairment Score +7 (mNIS+7). The Norfolk QoL-DN evaluates the patient’s experience with neuropathy and the mNIS+7 includes objective assessments of nerve damage.
Table 1. Clinical Primary Efficacy Results From Trial 1 (Comparison of AMVUTTRA Treatment in Study 1 to an External Placebo Control1)
| Endpoint2 | Baseline Mean (SD) | Change From Baseline to Month 9 LS Mean (SEM) | Treatment Difference LS Mean (95% CI) |
P‑value | ||
|---|---|---|---|---|---|---|
| AMVUTTRA N=122 |
Placebo1 N=77 |
AMVUTTRA N=122 |
Placebo1 N=77 |
|||
| mNIS+73 | 60.6 (36.0) | 74.6 (37.0) | -2.2 (1.4) | 14.8 (2.0) | -17.0 (-21.8, -12.2) |
<0.001 |
| Norfolk QoLDN3 | 47.1 (26.3) | 55.5 (24.3) | -3.3 (1.7) | 12.9 (2.2) | -16.2 (-21.7, -10.8) |
<0.001 |
| 10‑meter walk test (m/sec)4 | 1.01 (0.39) | 0.79 (0.32) | 0 (0.02) | -0.13 (0.03) | 0.13 (0.07, 0.19) |
<0.001 |
| mBMI5 | 1058 (234) | 990 (214) | 7.6 (7.9) | -60.2 (10.1) | 67.8 (43.0, 92.6) |
<0.001 |
Source: AMVUTTRA Prescribing Information
1 External placebo group from another randomized controlled trial (NCT01960348)
2 All endpoints analyzed using the analysis of covariance with multiple imputation method
3 A lower number indicates less impairment/fewer symptoms
4 A higher number indicates less disability/less impairment
5 mBMI: a nominal p value was reported; the endpoint was not formally tested for statistical significance; body mass index (kg/m2) multiplied by serum albumin (g/L).
Abbreviations: CI, confidence interval; LS, least squares; mBMI, modified body mass index; mNIS+7, modified Neuropathy Impairment Score +7; QoLDN, Quality of Life Diabetic Neuropathy; SD, standard deviation; SEM, standard error of the mean
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: AMVUTTRA worked similarly in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in how AMVUTTRA worked among races could not be determined.
- Age: AMVUTTRA worked similarly in patients younger and older than 65 years of age.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
Table 2 summarizes efficacy results by sex, race, and age.
Table 2. Subgroup Analyses of the Primary Endpoint, mNIS+7 (Trial 1)
| Subgroup | Baseline Mean | Change From Baseline at Month 9 LS Mean (SEM) | Treatment Difference LS Mean (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| N | AMVUTTRA | N | Placebo | AMVUTTRA | Placebo | ||
| Sex | |||||||
| Female | 43 | 64.6 | 19 | 53.9 | -5.5 (2.4) | 19.1 (3.9) | -24.5 (-33.8, -15.3) |
| Male | 79 | 77.9 | 58 | 64.2 | 0.2(1.7) | 14.1 (2.1) | -13.9 (-19.3, -8.5) |
| Race | |||||||
| White | 86 | 73.3 | 50 | 59.7 | -2.0 (1.5) | 12.7 (2.1) | -14.7 (-19.8, -9.7) |
| All others | 36 | 77.1 | 27 | 62.5 | -5.9 (3.3) | 16.8 (3.7) | -22.7 (-32.1, -13.3) |
| Age, years | |||||||
| <65 | 76 | 70.4 | 44 | 59.2 | -3.0 (1.6) | 12.3 (2.2) | -15.2 (-20.6, -9.9) |
| >65 | 46 | 80.2 | 33 | 62.9 | 0.6 (2.6) | 20.7 (3.2) | -20.1 (-28.4, -11.7) |
Source: Adapted from FDA Review
Abbreviations: CI, confidence interval; LS, least squares; mNIS+7, modified Neuropathy Impairment Score; SEM, standard error of the mean
What are the possible side effects?
AMVUTTRA may cause serious side effects including decreased vitamin A levels.
The most common side effects are joint pain, shortness of breath, and decreased vitamin A levels.
What are the possible side effects (results of trials used to assess safety)?
Table 3 summarizes adverse reactions in patients with hereditary transthyretin-mediated amyloidosis (Trial 1).
Table 3. Adverse Reactions Reported in at Least 5% of Patients Treated With AMVUTTRA (Trial 1)
| Adverse Reaction | AMVUTTRA N=122 % |
|---|---|
| Arthralgia* | 11 |
| Dyspnea* | 7 |
| Vitamin A decreased1 | 7 |
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The number of patients of races other than White was small; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar in patients younger and older than 65 years of age.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
Table 4 summarizes the occurrence of adverse events by subgroup.
Table 4. Overview of Side Effects by Age, Sex, and Race in Patients Treated with AMVUTTRA in Trial 1
| Demographic Variable | AMVUTTRA N=122 |
|
|---|---|---|
| All Patients n (%) |
Patients with Adverse Events n/Ns (%) |
|
| Age group, n (%) | ||
| <65 years | 76 (62.3) | 70/76 (92.1) |
| ≥65 years | 46 (37.7) | 44/46 (95.7) |
| Sex, n (%) | ||
| Female | 43 (35.2) | 41/43 (95.3) |
| Male | 79 (64.8) | 73/79 (92.4) |
| Race, n (%) | ||
| Asian | 21 (17.2) | 19/21 (90.5) |
| White | 86 (70.5) | 80/86 (93.0) |
| All others | 15 (12.3) | 15/15 (100) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 12 (9.8) | 11 (14.3) |
| Not Hispanic or Latino | 109 (89.3) | 65 (84.4) |
| Unknown | 1 (0.8) | 1 (1.3) |
Source: Adapted from FDA Review
Abbreviations: SD, standard deviation
Source: FDA reviewer’s analysis
Abbreviation: N, number of patients in the safety population; n, number of patients with given characteristic; Ns, total number of patients in each category.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.