Drug Trial Snapshot: XEGLYZE
HOW TO USE THIS SNAPSHOT
The information provided in Snapshots highlights who participated in the clinical trials that supported the FDA approval of this drug, and whether there were differences among sex, race and age groups. The “MORE INFO” bar shows more detailed, technical content for each section. The Snapshot is intended as one tool for consumers to use when discussing the risks and benefits of the drugs.
LIMITATIONS OF THIS SNAPSHOT:
Do not rely on Snapshots to make decisions regarding medical care. Always speak to your health provider about the risks and benefits of a drug. Refer to the XEGLYZE Package Insert for complete information.
XEGLYZE (abametapir)
(zeg’ lyze)
Dr. Reddy’s Laboratories
Approval date: July 24, 2020
DRUG TRIALS SNAPSHOT SUMMARY:
What is the drug for?
XEGLYZE is a drug used to treat head lice in patients 6 months of age and older.
How is this drug used?
XEGLYZE is a lotion applied once to dry hair.
What are the benefits of this drug?
About 80% of patients treated with XEGLYZE were free of live lice in comparison to about 50% of patients who were treated with placebo.
What are the benefits of this drug (results of trials used to assess efficacy)?
The table below summarizes efficacy results for the evaluated patients in Trials 1 and 2. Efficacy was assessed as the proportion of index subjects who were treated with a single 10-minute application and were free of live lice at all follow-up visits on Days 1, 7, and 14.
Table 1. Proportion of Index Patients Free of Live Lice at All Visits Days 1 Through 14
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
|
XEGLYZE |
Vehicle |
XEGLYZE |
Vehicle |
|
Treatment Success |
43 (81.1%) |
28 (50.9%) |
45 (81.8%) |
25 (47.2%) |
XEGLYZE Prescribing Information
Were there any differences in how well the drug worked in clinical trials among sex, race and age?
- Sex: The majority of patients in the trials were females. XEGLYZE appeared to be similarly effective in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in how XEGLYZE worked among races could not be determined.
- Age: XEGLYZE worked similarly in all tested age groups.
Were there any differences in how well the drug worked in clinical trials among sex, race, and age groups?
The table below summarizes efficacy results by sex, race, and age subgroup.
Table 2. Subgroup Efficacy Analyses
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
|
XEGLYZE N=53 |
Vehicle N=55 |
XEGLYZE N=55 |
Vehicle N=53 |
|
Sex |
||||
|
Male |
5/5 (100%) |
6/10 (60%) |
4/7 (57%) |
6/10 (60%) |
|
Female |
38/48 (79%) |
22/45 (49%) |
41/48 (85%) |
19/43 (44%) |
|
Race |
||||
|
White |
41/50 (82%) |
28/55 (51%) |
44/51 (86%) |
23/49 (47%) |
|
Black or African American |
1/2 (50%) |
- |
- |
1/2 (50%) |
|
Other |
1/1 (100%) |
- |
1/4 (25%) |
1/2 (50%) |
|
Age Group |
||||
|
6 months – 4 years |
10/11 (91%) |
7/11 (64%) |
5/7 (71%) |
4/11 (36%) |
|
4-12 years |
28/36 (78%) |
18/39 (46%) |
33/41 (80%) |
15/36 (42%) |
|
12-18 years |
3/4 (75%) |
1/3 (33%) |
2/2 (100%) |
4/4 (100%) |
|
≥18-65 years |
2/2 (100%) |
2/2 (100%) |
5/5 (100%) |
2/2 (100%) |
|
≥ 65 years |
- |
- |
- |
- |
FDA Statistical Review
What are the possible side effects?
XEGLYZE contains benzyl alcohol which may cause serious injury if accidentally ingested or used in patients younger than 6 months.
The most common side effects of XEGLYZE are skin redness, rash, skin burning sensation, skin inflammation, vomiting, eye irritation, skin itching, and hair color changes.
What are the possible side effects (results of trials used to assess safety)?
The table below provides adverse reactions that occurred in at least 1% of patients in the XEGLYZE group and at a greater frequency than in the vehicle (placebo) group.
Table 3. Adverse Reactions Occurring in ≥ 1% of the XEGLYZE Group and at a Greater Frequency than in the Vehicle Group (Trials 1 and 2)
|
Adverse Reactions |
XEGLYZE N=349 (%) |
Vehicle N=350 (%) |
|---|---|---|
|
Erythema |
14 (4.0) |
6 (2) |
|
Rash |
11 (3.2) |
8 (2.3) |
|
Skin burning sensation |
9 (2.6) |
0 (0.0) |
|
Contact dermatitis |
6 (1.7) |
4 (1.1) |
|
Vomiting |
6 (1.7) |
2 (0.6) |
|
Eye irritation |
4 (1.2) |
2 (0.6) |
|
Hair color changes |
3 (1) |
0 (0.0) |
The number and percentage of patients who developed local adverse reactions after treatment are presented in Table 4.
Table 4. Monitored Local Adverse Reactions with New Onset on Day 1 Post-Treatment (Trials 1 and 2)
|
Adverse Reactions |
XEGLYZE (%)* |
Vehicle (%)* |
|---|---|---|
|
Scalp Erythema/Edema |
11 (3.2) |
5 (1.4) |
|
Scalp Pruritus |
2 (1.4) |
1 (0.7) |
|
Eye Irritation |
6 (1.7) |
5 (1.4) |
* For the calculation of the percentages, the denominators are the number of subjects who did not have the monitored local adverse reaction at baseline.
XEGLYZE Prescribing Information
Were there any differences in side effects among sex, race and age?
- Sex: The occurrence of side effects was similar in males and females.
- Race: The majority of patients were White. The number of patients in other races was limited; therefore, differences in the occurrence of side effects among races could not be determined.
- Age: The occurrence of side effects was similar among tested age groups.
Were there any differences in side effects of the clinical trials among sex, race, and age groups?
The tables below summarize the occurrence of the most common adverse reactions, erythema and rash, by sex and age subgroups. Race was not analyzed because of White race predominance.
Table 5. Subgroup Analysis of Selected Adverse Reactions by Sex
|
Adverse Reaction
|
Males |
Females |
||
|---|---|---|---|---|
|
XEGLYZE N=51 |
Vehicle N=64 |
XEGLYZE N=298 |
Vehicle N=286 |
|
|
Erythema, % |
6 |
2 |
4 |
2 |
|
Rash, % |
0 |
2 |
4 |
3 |
Table 6. Subgroup Analysis of Selected Adverse Reactions by Age
|
Adverse Reaction
|
2-4 years (%) |
4-12 years (%) |
12-18 years |
≥18 years |
||||
|---|---|---|---|---|---|---|---|---|
|
XEGLYZE N=15 |
Vehicle N=24 |
XEGLYZE N=166 |
Vehicle N=174 |
XEGLYZE N=56 |
Vehicle N=52 |
XEGLYZE N=105 |
Vehicle N=92 |
|
|
Erythema, % |
7 |
0 |
5 |
2 |
4 |
2 |
3 |
1 |
|
Rash, % |
7 |
0 |
4 |
4 |
2 |
0 |
3 |
1 |
Adapted from FDA Clinical Review
WHO WAS IN THE CLINICAL TRIALS?
Who participated in the clinical trials?
The FDA approved XEGLYZE based on evidence from two identical clinical trials of 699 patients with head lice. The trials were conducted at 14 sites in the United States.
The population that provided data for the evaluation of side effects (safety population) is presented below. Demographics of the patients who provided data for evaluation of benefits are presented in Table 8, under the MORE INFO section.
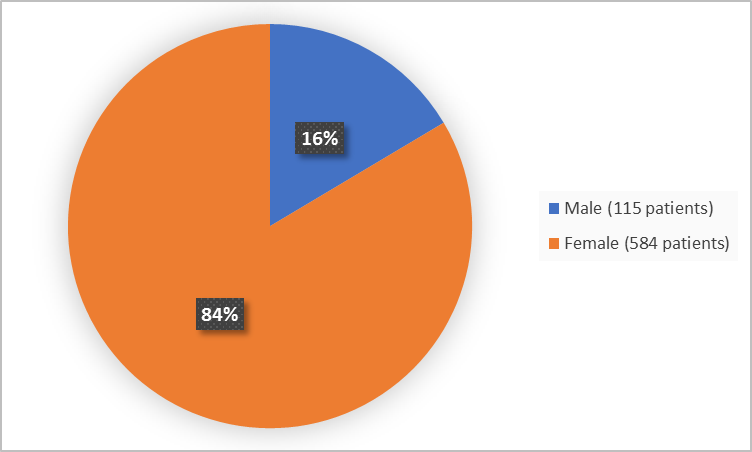
Figure 1 summarizes how many males and females were in the clinical trials used to evaluate safety.
Figure 1. Baseline Demographics by Sex
FDA Clinical Review
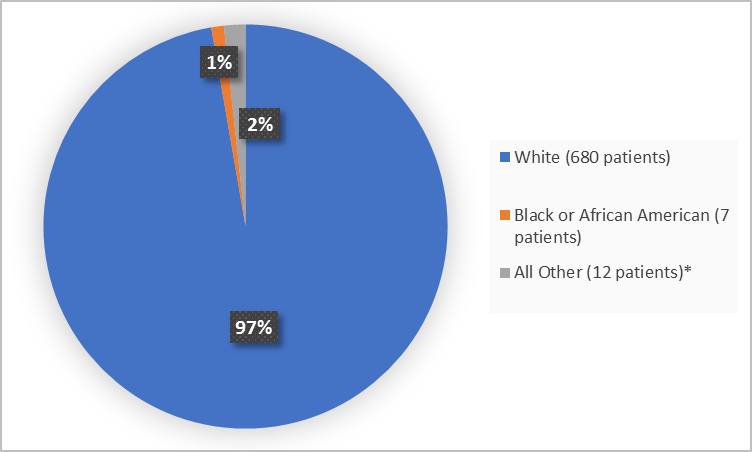
Figure 2 summarizes the percentage of patients by race in the clinical trials used to evaluate safety.
Figure 2. Baseline Demographics by Race
*Other includes American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander
FDA Clinical Review
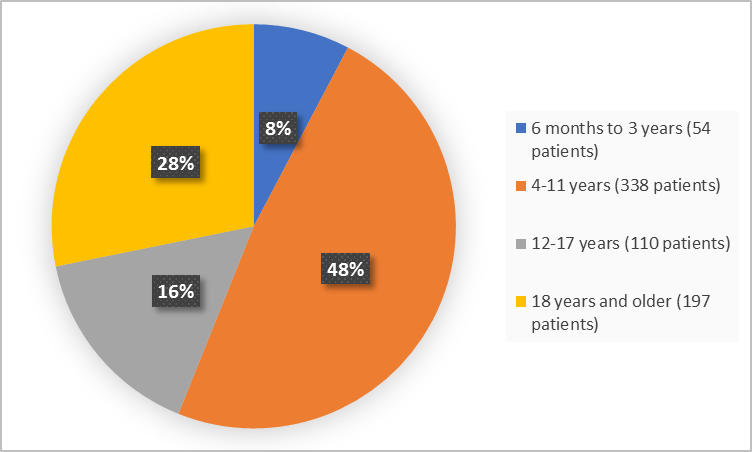
Figure 3 summarizes the percentage of patients by age in the clinical trials used to evaluate safety.
Figure 3. Baseline Demographics by Age
FDA Clinical Review
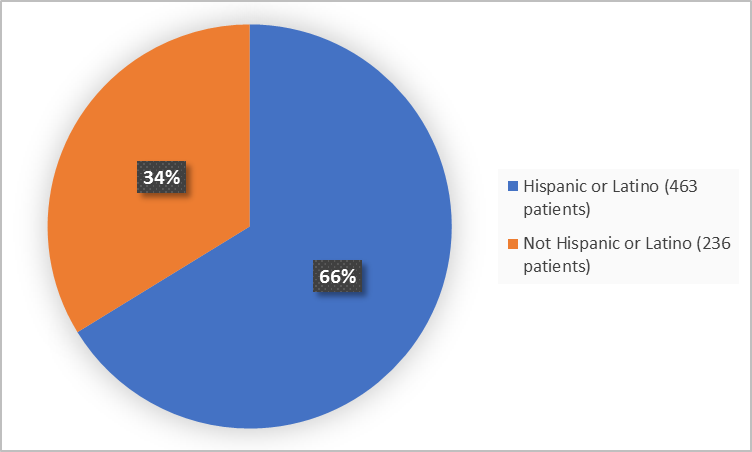
Figure 4 summarizes the percentage of patients by ethnicity in the clinical trials used to evaluate safety.
Figure 4. Baseline Demographics by Ethnicity
FDA Clinical Review
Who participated in the trials?
The tables below summarize demographics of the safety population and the intent-to-treat (ITT) population.
Table 7. Baseline Demographics (Safety Population)
|
XEGLYZE (N=349) |
Placebo (N=350) |
Total (N=699) |
|
|---|---|---|---|
|
Sex, n(%) |
|||
|
Male |
51 (14.6) |
64 (18.3) |
115 (16.5) |
|
Female |
298 (85.4) |
286 (81.7) |
584 (83.5) |
|
Race n(%) |
|||
|
White |
334 (95.7) |
346 (98.9) |
680 (97.3) |
|
Black or African American |
4 (1.1) |
3 (0.8) |
7 (1) |
|
American Indian or Alaska Native |
3 (0.9) |
0 |
3 (0.4) |
|
Native Hawaiian or Other Pacific Islander |
0 |
1 (0.3) |
1 (<1) |
|
Other |
8 (2.3) |
0 |
8 (1.1) |
|
Age (years) |
|||
|
Range |
0.5, 60 |
1.1, 61 |
0.5, 61 |
|
Age Group n(%) |
|||
|
6 months to <4 years |
22 (6.3) |
32 (9.1) |
54 (7.7) |
|
4 to <12 years |
165 (47.3) |
173 (49.4) |
338 (48.3) |
|
12 to <18 years |
57 (16.3) |
53 (15.1) |
110 (15.7) |
|
18 years and older |
105 (30.1) |
92 (26.3) |
197 (28.2) |
|
Ethnicity n(%) |
|||
|
Hispanic or Latino |
230 (65.9) |
233 (66.6) |
463 (66.2) |
|
Non-Hispanic or |
119 (34.1) |
117 (33.3) |
236 (33.8) |
|
Region n(%) |
|||
|
United States |
349 (100) |
350 (100) |
699 (100) |
FDA Clinical Review
Table 8. Baseline Demographics of Index Patients, Intent-to Treat (ITT) Population
|
|
Trial 1 |
Trial 2 |
||
|---|---|---|---|---|
|
|
XEGLYZE |
Vehicle |
XEGLYZE |
Vehicle |
|
Sex, n(%) |
||||
|
Male |
5 (9%) |
10 (18%) |
7 (13%) |
10 (19%) |
|
Female |
48 (91%) |
45 (82%) |
48 (87%) |
43 (81%) |
|
Race, n(%) |
||||
|
White |
50 (94%) |
55 (100%) |
51 (93%) |
49 (92%) |
|
Black or African American |
2 (4%) |
0 (0%) |
0 (0%) |
2 (4%) |
|
Other |
1 (2%) |
0 (0%) |
4 (7%) |
2 (4%) |
|
Age |
||||
|
Mean (SD) |
7.5 (4.2) |
7.4 (6.7) |
9.8 (10.5) |
7.8 (7.7) |
|
Median |
6.8 |
6.0 |
7.0 |
6.5 |
|
Range |
0.5-19.2 |
1.2-49.1 |
1.6-58.5 |
1.1-56.9 |
|
Age Group, n(%) |
||||
|
6 months – 4 years |
11 (21%) |
11 (20%) |
7 (13%) |
11 (21%) |
|
4-12 years |
36 (68%) |
39 (71%) |
41 (93%) |
36 (57%) |
|
12-18 years |
4 (8%) |
3 (5%) |
2 (4%) |
4 (8%) |
|
≥ 18-65 years |
2 (4%) |
2 (4%) |
5 (9%) |
2 (4%) |
|
≥ 65 years |
0 (0%) |
0 (0%) |
0 (0%) |
0 (0%) |
FDA Statistical Review
How were the trials designed?
The benefit and side effects of XEGLYZE were evaluated in two clinical trials that enrolled patients with head lice who were at least 6 months old.
About half of all enrolled patients was randomly assigned to XEGLYZE and the other half to placebo. XEGLYZE lotion or placebo lotion were applied once as a 10-minute treatment to infested hair. The benefit of XEGLYZE in comparison to placebo was assessed after 1, 7 and 14 days by comparing the counts of patients in each group who were free of live lice.
How were the trials designed?
The safety and efficacy of XEGLYZE was established in two multi-center, randomized, double-blind, vehicle-controlled trials. Enrolled patients were 6 months of age and older with head lice infestation. All patients received a single application of either XEGLYZE or vehicle.
For the evaluation of efficacy, the youngest patient from each household was considered to be the index patient of the household. Efficacy was assessed as the proportion of index patients who were treated with a single 10-minute application and were free of live lice at all follow-up visits on Days 1, 7, and 14.
GLOSSARY
CLINICAL TRIAL: Voluntary research studies conducted in people and designed to answer specific questions about the safety or effectiveness of drugs, vaccines, other therapies, or new ways of using existing treatments.
COMPARATOR: A previously available treatment or placebo used in clinical trials that is compared to the actual drug being tested.
EFFICACY: How well the drug achieves the desired response when it is taken as described in a controlled clinical setting, such as during a clinical trial.
PLACEBO: An inactive substance or “sugar pill” that looks the same as, and is given the same way as, an active drug or treatment being tested. The effects of the active drug or treatment are compared to the effects of the placebo.
SUBGROUP: A subset of the population studied in a clinical trial. Demographic subsets include sex, race, and age groups.