Animal Drug Safety-Related Labeling Changes
This online resource provides information on recently approved safety-related labeling changes for animal drugs from January 2021 forward in an effort to improve transparency and communication with veterinarians and the public.
When adverse events are reported or safety concerns are identified for an animal drug, the FDA may work with the sponsor to revise the labeling to reflect this information. This webpage includes safety-related labeling changes initiated by the company or the FDA. Safety-related labeling changes include updates or revisions to any section of the label containing safety information. Awareness of these safety-related labeling changes is essential for the safe use and administration of FDA-approved animal products.
There is often a lag between the approval of labeling changes and the new labeling becoming available in the marketplace. You can check this webpage for recent safety-related labeling changes as it will be updated on a regular basis when changes to the drug labeling are approved by the FDA.
For more information about the most current labeling for a particular animal drug, veterinarians should reach out to the drug’s sponsor.
Disclaimer
This webpage reflects recently approved safety-related labeling changes for FDA approved brand name new animal drugs and is also applicable to any generics of those products. Generic new animal drugs are copies of brand name new animal drugs approved by the FDA and must provide the same labeling information (e.g., Indications, Warnings, Cautions, etc.). Therefore, the safety-related labeling changes reflected on this webpage also apply to generic new animal drugs.
The labeling for an approved animal drug, such as its carton or package insert, might not reflect the changes for a year or more in the marketplace as the drug company distributes its inventory of the drug with labeling printed prior to the approval of the labeling changes.
Any reference to a commercial product, process, service, or company is not an endorsement or recommendation by the U.S. government, the Department of Health and Human Services, the FDA or any of its components. The FDA is not responsible for the contents of any non-FDA website referenced by or linked to the agency's website.
Animal Drug Safety-Related Labeling Changes 2024-present:
Indications:
Bravecto kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick), and Rhipicephalus sanguineus (brown dog tick)] for 12 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.
Bravecto is also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for 8 weeks in dogs and puppies 6 months of age and older, and weighing 4.4 pounds or greater.
Summary of Changes:
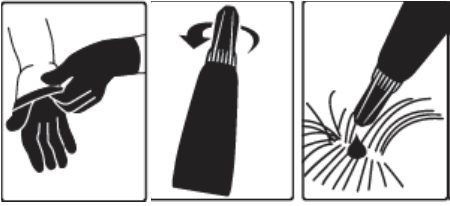
- The Dosage and Administration section was revised to add language instructing the veterinarian to demonstrate to the client how to properly apply the product and inform users that gloves should be worn. Language describing that the cap is designed to stay on the tube was underlined to emphasize this information. The pictogram was also revised to demonstrate putting on gloves.
- The Human Warnings section was revised to provide information regarding accidental ocular exposure and contact with the skin.
- The Precautions section was revised to add language about adverse events being reported in breeding females following use of Bravecto (fluralaner) Chews.
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
The following safety-related changes were made to the labeling:
Dosage and Administration
(Additions and/or revisions are underlined)
…
A veterinarian or veterinary technician should demonstrate or instruct the pet owner regarding the appropriate technique for applying Bravecto topically to dogs prior to first use.
Step 1: Immediately before use, open the pouch and remove the tube. Put on gloves. Hold the tube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counter clockwise one full turn. The cap is designed to stay on the tube for dosing and should not be removed. The tube is open and ready for application when a breaking of the seal is felt.
…
Warnings
(Additions and/or revisions are underlined)
Human Warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not contact or allow children to contact the application site until 2 hours post application.
Keep the product in the original packaging until use in order to prevent children from getting direct access to the product.
Do not eat, drink, or smoke while handling the product. Avoid contact with skin and eyes. If contact with eyes occurs, then flush eyes slowly and gently with water. If wearing contact lenses, eyes should be rinsed first, then remove contact lenses and continue rinsing, then seek medical advice immediately. Wash hands and contacted skin thoroughly with soap and water immediately after use of the product. If the product accidentally contacts skin and a sticky residue persists after washing, rubbing alcohol (70% isopropyl alcohol) can be applied to the area to remove the residue.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
Precautions
(Additions and/or revisions are underlined)
…
The safety of Bravecto (fluralaner topical solution) in breeding, pregnant, and lactating dogs was evaluated using the oral formulation (see Animal Safety and Clinical Pharmacology sections). Adverse events have been reported following use of Bravecto (fluralaner) Chews in breeding females.
Before use in breeding female dogs, refer to Post-Approval Experience and Animal Safety sections.
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2025)
The following adverse events are based on post-approval adverse drug experience reporting for Bravecto (fluralaner topical solution). Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs are listed in decreasing order of reporting frequency:
Lethargy, vomiting, application site disorders (including alopecia, pruritus, and erythema), behavioral changes (including hyperactivity and vocalization), anorexia/decreased appetite, seizure, diarrhea, generalized pruritus, tremors, and ataxia.
The following reproductive adverse events have been reported following use of Bravecto (fluralaner) Chews: birth defects (including limb deformities and cleft palate), stillbirth, and abortion.
Indications:
Complete Type C Medicated Feeds: For increased rate of weight gain, improved feed efficiency and increased carcass leanness in growing beef steers and heifers fed in confinement for slaughter during the last 28 to 42 days on feed. Not for use in cattle intended for breeding.
Type C Top-Dress Medicated Feeds: For increased rate of weight gain and improved feed efficiency in growing beef steers and heifers fed in confinement for slaughter during the last 28 to 42 days on feed. Not for use in cattle intended for breeding. Note: Increased carcass leanness is not an approved indication for use with Top-Dress Feeding.
Summary of Changes:
- A new Animal Safety Warnings subsection was added to the Warnings section of the label for the Type A medicated article to add information regarding behavioral signs in cattle fed ractopamine hydrochloride.
- A new Additional Recommendations section was added following the Warnings section of the label for the Type A medicated article to direct users to additional tools and resources to help mitigate potential adverse events.
- The Type B and C Medicated Feed Blue Bird labels were updated to reflect the revisions to the Type A medicated article labeling.
The following safety-related changes were made to the labeling:
Warnings
(Newly added subsection)
Animal Safety Warnings
Behavioral signs such as agitation and decreased feed consumption have been reported in cattle fed ractopamine hydrochloride.
Additional Recommendations
(Newly added section)
See the Beef Quality Assurance (BQA) recommendations for best practices in cattle care and handling during Optaflexx feeding, transport, and marketing to help mitigate the behavioral signs stated in the Animal Safety Warnings section above.
Indications:
For management of weight loss in cats with chronic kidney disease.
Summary of Changes:
- The Warnings section was revised to add a User Safety Warnings subsection, including a statement informing users to wash their hands after administering the product as it can be dermally absorbed, and an Animal Safety Warnings subsection, including a statement to keep ELURA in a secure location.
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
The following safety-related changes were made to the labeling:
Warnings
(Newly added subsections. Additions and/or revisions are underlined)
User Safety Warnings
Not for use in humans. Keep this drug, including used syringes, out of reach of children. Wash hands immediately after use as this product may be dermally absorbed. Consult a physician in case of accidental ingestion by humans. To obtain a Safety Data Sheet(s), contact Elanco US Inc. at 1-888-545-5973.
For oral use in cats only.
Animal Safety Warnings
…
Keep ELURA in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2025)
The following adverse events are based on post-approval adverse drug experience reporting for ELURA. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in cats are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
Gastrointestinal: hypersalivation, vomiting
General: lethargy, anorexia, recumbency, weakness
Behavioral: unusual behaviors, hiding, vocalization, hyperactivity
Cardiovascular: bradycardia, hypotension
Neurological: loss of consciousness, sedation
Respiratory: dyspnea
Indications:
ENTYCE (capromorelin oral solution) is indicated for appetite stimulation in dogs.
Summary of Changes:
- The Dosage and Administration section was revised to add instructions, accompanied by illustrations, describing how to draw up the solution from the bottle and a statement informing users to wash their hands after administering the product.
- The Warnings section was revised to add a User Safety Warnings subsection, including a statement informing users to wash their hands after administering the product as it can be dermally absorbed, and an Animal Safety Warnings subsection, including a statement to keep ENTYCE in a secure location.
- The Precautions section was revised to add the recommendation that ENTYCE be used with caution in dogs with cardiac disease, severe dehydration, or diabetes mellitus.
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
The following safety-related changes were made to the labeling:
Dosage and Administration:
(Additions and/or revisions are underlined)
Administer ENTYCE orally at a dose of 3 mg/kg (1.4 mg/lb) body weight once daily.
To administer ENTYCE follow the written instructions below and see illustrations 1 through 4 for administration steps.
- Gently shake the bottle.
- Remove the bottle cap and insert the provided dosing syringe firmly into the opening of the bottle.
- Turn the bottle upside down and withdraw the appropriate volume of solution. Return the bottle to the upright position before removing the syringe, and replace the cap.
- Administer the solution with the syringe into the dog's mouth.
Rinse the syringe and plunger with water between treatment doses. Leave the syringe and plunger apart to dry. Wash hands immediately after use.
The effectiveness of ENTYCE has not been evaluated beyond 4 days of treatment in the clinical field study (See Effectiveness).
Warnings
(Newly added subsections. Additions and/or revisions are underlined)
User Safety Warnings:
Not for use in humans. Keep this drug, including used syringes, out of reach of children. Wash hands immediately after use as this product may be dermally absorbed. Consult a physician in case of accidental ingestion by humans. To obtain a Safety Data Sheet(s), contact Elanco US Inc. at 1-888-545-5973.
For use in dogs only.
Animal Safety Warnings:
Keep ENTYCE in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions
(Additions and/or revisions are underlined)
…
Use with caution in dogs that may have cardiac disease or severe dehydration. ENTYCE can cause transient decreases in heart rate and blood pressure following dose administration. Some dogs may exhibit clinical signs of bradycardia or hypotension following administration of ENTYCE (See Post-Approval Experience).
Use with caution in dogs with diabetes mellitus as hyperglycemia has been reported following administration of ENTYCE (See Post-Approval Experience).
…
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2025):
The following adverse events are based on post-approval adverse drug experience reporting for ENTYCE. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in dogs are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
Gastrointestinal: vomiting, hypersalivation, diarrhea
General: lethargy, polydipsia, weakness, hyperglycemia, recumbency
Behavioral: Unusual behaviors (some of these behaviors were related to avoidance of medication), vocalization, hyperactivity
Neurological: ataxia, loss of consciousness, disorientation, sedation
Respiratory: panting, dyspnea
Cardiovascular: bradycardia, hypotension
Indications:
For increased rate of weight gain, improved feed efficiency and increased carcass leanness in finishing swine, weighing at least 150 lbs, fed a complete ration containing at least 16% crude protein for the last 45 to 90 lbs of gain prior to slaughter. Not for use in swine intended for breeding.
Summary of Changes:
- A new Animal Safety Warnings subsection was added to the Warnings section of the label for the Type A medicated articles to incorporate information previously presented in the Caution section. This subsection was updated to include information regarding lameness and behavioral signs in pigs.
- A new Additional Recommendations section was added following the Warnings section of the label for Type A medicated articles to direct users to additional tools and resources to help mitigate potential adverse events.
- The Type B and C Medicated Feed Blue Bird labels were updated to reflect the revisions to the Type A medicated article labeling.
The following safety-related changes were made to the labeling:
Warnings
(Newly added subsection with additions underlined)
Animal Safety Warnings
Ractopamine hydrochloride use may increase the number of injured, lame, and/or fatigued pigs during marketing. Behavioral signs such as hyperactivity, anxiety, and aggression have been reported in pigs fed ractopamine hydrochloride.
Additional Recommendations
(Newly added section.)
To help mitigate the signs identified in the Animal Safety Warnings section, see the Pork Quality Assurance (PQA Plus) and Transport Quality Assurance (TQA) recommendations for best practices in swine care during handling, transport, and marketing.
Indications:
Zenrelia is indicated for control of pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age.
Summary of Changes:
- The Boxed Warning was revised to remove the language regarding risk of fatal vaccine-induced disease from modified live virus vaccines.
- A statement instructing that, due to the risk of infections, dogs should be up to date on vaccinations prior to starting Zenrelia was revised and relocated from the Precautions section to the Animal Safety Warnings subsection of the Warnings section.
- The Animal Safety Warnings subsection was revised to remove the language regarding risk of fatal vaccine-induced disease from modified live virus vaccines.
- The Animal Safety Warnings subsection was revised to add the risk of increased susceptibility to adenoviral hepatitis and pancreatitis to the statement about monitoring for the development of infections. This entire statement was bolded to emphasize this information.
Note: The Vaccine Response Study subsection of the Target Animal Safety section was also revised to remove the language regarding vaccine-induced disease and to include new information about susceptibility to adenoviral infection.
The following safety-related changes were made to the labeling:
Boxed Warning
(Removed language is noted by strikethrough)
WARNING: VACCINE-INDUCED DISEASE AND INADEQUATE IMMUNE RESPONSE TO VACCINES
Based on results of the vaccine response study, dogs receiving Zenrelia are at risk of fatal vaccine-induced disease from modified live virus vaccines and an inadequate immune response to vaccines. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination (see Warnings and Target Animal Safety).
Animal Safety Warnings
(Removed language is noted by strikethrough and additions and/or revisions are underlined)
Due to the risk of infections, dogs should be up to date on vaccinations prior to starting Zenrelia (see Target Animal Safety).
Due to the risk of fatal vaccine-induced disease from modified live virus vaccines and an inadequate immune response to vaccines, including rabies vaccines, do not administer vaccines to a dog receiving Zenrelia. Discontinue Zenrelia for at least 28 days to 3 months prior to vaccination and withhold Zenrelia for at least 28 days after vaccination (see Target Animal Safety).
Dogs should be monitored for the development of infections because Zenrelia may increase susceptibility to infections, including adenoviral hepatitis and pancreatitis, demodicosis, interdigital furunculosis, coccidiosis, and pneumonia, and exacerbation of subclinical or uncomplicated infections (see Target Animal Safety and Adverse Reactions).
…
Indications:
SIMPARICA TRIO is indicated for the prevention of heartworm disease caused by Dirofilaria immitis and for the treatment and control of roundworm (immature adult and adult Toxocara canis and adult Toxascaris leonina) and hookworm (L4, immature adult, and adult Ancylostoma caninum and adult Uncinaria stenocephala) infections. SIMPARICA TRIO kills adult fleas (Ctenocephalides felis) and is indicated for the treatment and prevention of flea infestations, the prevention of Dipylidium caninum (tapeworm) infections as a direct result of killing Ctenocephalides felis vector fleas on the treated dog, and the treatment and control of tick infestations with Amblyomma americanum (lone star tick), Amblyomma maculatum (Gulf Coast tick), Dermacentor variabilis (American dog tick), Ixodes scapularis (black-legged tick), Rhipicephalus sanguineus (brown dog tick), and Haemaphysalis longicornis (Asian longhorned tick) for one month in dogs and puppies 8 weeks of age and older, and weighing 2.8 pounds or greater. SIMPARICA TRIO is indicated for the prevention of Borrelia burgdorferi infections as a direct result of killing Ixodes scapularis vector ticks.
Summary of Changes:
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
Note: A new indication was added for the prevention of Dipylidium caninum (tapeworm) infections as a direct result of killing Ctenocephalides felis vector fleas on the treated dog (see above). This new indication is listed here for informational purposes only; it does not affect the safe use of the product. Information related to this new indication was also added to the Dosage and Administration and Effectiveness sections, which are not presented here.
The following safety-related changes were made to the labeling:
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2024)
The following adverse events are based on post-approval adverse drug experience reporting for SIMPARICA TRIO. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs are listed in decreasing order of reporting frequency:
Vomiting, diarrhea (with and without blood), seizure, lethargy, anorexia, muscle tremor, ataxia, non-specific behavioral changes, and pruritus.
Indications:
LIBRELA is indicated for the control of pain associated with osteoarthritis in dogs.
Summary of Changes:
- The Dosage and Administration section was updated to direct veterinarians to provide and discuss the Client Information Sheet with dog owners.
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
- An Information for Dog Owners section was added to advise veterinarians to discuss the Client Information Sheet with dog owners prior to administering LIBRELA, emphasizing potential adverse drug events and the development of an exercise plan for return to activity.
- A Client Information Sheet was added to provide information about LIBRELA to dog owners, emphasizing safety and risk information and post-approval experience.
The following safety-related changes were made to the labeling:
Dosage and Administration
(Additions and/or revisions are underlined)
Always provide the Client Information Sheet and discuss potential adverse drug events with the dog owner prior to administering each injection of LIBRELA (see Post-Approval Experience and Information for Dog Owners).
…
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2025)
The following adverse events are based on post-approval adverse drug experience reporting for LIBRELA. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in dogs are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
Neurological: ataxia, seizures, paresis, proprioception deficits, paralysis
General: anorexia, lethargy, recumbency
Urinary: polydipsia, polyuria/pollakiuria, urinary incontinence
Gastrointestinal: vomiting, diarrhea
Musculoskeletal: muscle weakness, muscle tremors, lameness
In some cases, death (including euthanasia) has been reported as an outcome of the adverse events listed above.
Information for Dog Owners
(Newly added section)
Always provide and review the Client Information Sheet with the dog owner prior to each injection of LIBRELA. Advise owners of the adverse reactions that may occur following the administration of LIBRELA including balance problems, trouble walking, weakness, trouble standing, seizures, drinking more, urinating more, loss of bladder control, vomiting, and diarrhea. Owners should be advised to contact their veterinarian if any of these signs or other signs of illness are observed. Advise dog owners that serious adverse reactions can occur and result in death or euthanasia (see Adverse Reactions and Post-Approval Experience).
Where a dog has not been able to exercise prior to treatment with LIBRELA, there is an increased risk of injury if the dog becomes more active after being treated with LIBRELA. Discuss an exercise plan with the dog owner to return the dog to activity.
Client Information Sheet
(Newly added labeling component)
You should read this information before starting your dog on LIBRELA and review it with your veterinarian each time your dog receives an injection as there may be new information. This Client Information Sheet provides a summary and does not take the place of the instructions from your veterinarian. Talk to your veterinarian if you do not understand any of this information or you want more information about LIBRELA.
What is LIBRELA?
LIBRELA is a drug used for the control of pain associated with osteoarthritis (arthritis) in dogs. It is a commercially produced monoclonal antibody (a protein) which binds to another protein in your dog called nerve growth factor (NGF). NGF can be elevated in the joints of dogs with arthritis and is associated with the transmission of the pain sensation to the brain. When LIBRELA binds to NGF, it reduces the effect of NGF.
How is LIBRELA given to my dog?
LIBRELA is given as a once-a-month subcutaneous injection (under the skin) by your veterinarian.
What can I expect from my dog’s treatment with LIBRELA?
- While LIBRELA does not treat, slow, or reverse the progression of arthritis, LIBRELA can alleviate arthritis pain for 1-month post-injection.
- It may take two injections (given one month apart) to notice a reduction in your dog’s pain.
- If your dog does not respond to treatment as desired after the second injection or does not continue to respond after multiple injections talk to your veterinarian about alternative treatments.
- If your dog has not been able to exercise prior to treatment, discuss an exercise plan with your veterinarian to prevent injury as your dog returns to activity.
- Discuss your dog’s response to LIBRELA with your veterinarian prior to each injection to determine if LIBRELA is working for your dog and to decide whether your dog should continue to receive LIBRELA.
What should I discuss with my veterinarian before my dog receives each injection of LIBRELA?
Talk with your veterinarian about:
- The risks and benefits of using LIBRELA.
- Your dog’s previous or current medical problems, especially:
- balance problems
- trouble standing or walking
- seizures
- urinary problems
- All medications your dog is taking, including prescription drugs, over the counter drugs, heartworm preventatives, flea and tick medications, and vitamins and supplements, including herbal medications or homeopathic products.
- Any previous, or current, monoclonal antibody therapy your dog has received.
What dogs should not be given LIBRELA?
Your dog should not be given LIBRELA:
- If your dog is less than 12 months of age.
- If you have plans to breed your dog or they are pregnant or nursing.
- If your dog has new health problems or their health has recently changed, because further tests may be recommended prior to starting or continuing LIBRELA.
What are some possible side effects of LIBRELA?
The following side effects have been seen in dogs given LIBRELA:
- Balance problems or trouble walking
- Weakness or trouble standing
- Paralysis
- Seizures
- Drinking more
- Urinating more
- Loss of bladder control
- Vomiting or diarrhea
Serious side effects can occur, with or without warning, and in some situations may result in death.
Contact your veterinarian if you observe any of these changes in your dog or your dog has any other changes that concern you.
This Client Information Sheet provides a summary of information about LIBRELA. If you have any questions or concerns about LIBRELA, talk to your veterinarian.
To report side effects call Zoetis at 1-888-963-8471. For additional information about reporting side effects, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
Indications:
STELFONTA injection is indicated for use in dogs for the treatment of:
- non-metastatic cutaneous mast cell tumors
- non-metastatic subcutaneous mast cell tumors located at or distal to the elbow or the hock.
Summary of Changes:
- The Dosage and Administration section was updated to highlight the importance of appropriate administration of the required concomitant medications and to clarify the dosing instructions for both the concomitant medications and STELFONTA.
- Safety and risk information was updated in the Animal Safety Warnings, Precautions, and Information for Dog Owners sections to reflect post-approval experience, including extensive wound formation, limb amputation, and death.
- A Post-Approval Experience subsection was added to the Adverse Reaction section.
- The Client Information Sheet was updated to emphasize the potential for serious side effects and reflect the new safety and risk information and post-approval experience information, including diagrams illustrating extensive wound formation.
In addition, a fillable medication schedule and explanatory language were added to facilitate correct administration of concomitant medications.
The following safety-related changes were made to the labeling:
Dosage and Administration
(Additions and/or revision are underlined)
ALWAYS PROVIDE THE CLIENT INFORMATION SHEET TO THE DOG OWNER BEFORE DOSE ADMINISTRATION.
Carefully consider the potential benefits and risks of STELFONTA before deciding to use STELFONTA.
It is crucial to follow the dosing and administration instructions to use the product safely and effectively (see Boxed Warning, Animal Warnings, Precautions, Clinical Pharmacology).
Concomitant medications
Administer the following medications to decrease the potential for severe systemic adverse reactions from mast cell degranulation (see Effectiveness). Do not underdose concomitant medications and confirm that the dog owner administered the medications as prescribed prior to the day of STELFONTA treatment.
- Corticosteroid (oral prednisone or prednisolone at anti-inflammatory dose): Start medication 2 days prior to STELFONTA treatment at a dose of 0.5 mg/kg orally every 12 hours for 7 days (2 days prior, the day of treatment, and 4 days after treatment), then 0.5 mg/kg orally every 24 hours for an additional 3 days (10 days total).
- H1 receptor blocking agent (oral diphenhydramine): Start medication on the day of STELFONTA treatment at a dose of 2 mg/kg orally every 12 hours and continue for a total of 8 days (the day of treatment and 7 days after treatment).
- H2 receptor blocking agent (oral famotidine): Start medication on the day of STELFONTA treatment at a dose of 0.5 mg/kg orally every 12 hours and continue for a total of 8 days (the day of treatment and 7 days after treatment).
- Fill out the medication schedule (drug name, dose, route of administration, date) on the Client Information Sheet to help the dog owner administer these medications correctly.
Consider administering analgesic medications prior to, during, and after treatment with STELFONTA.
Dosing Instructions
Administer STELFONTA as an intratumoral injection at a dose of 0.5 mL per cm3 of tumor volume, as determined by the following calculations:
STEP 1. Calculate Tumor Volume:
- Measure the tumor dimensions (Length, Width and Height) with calipers on the day of STELFONTA injection.
- Determine the Tumor Volume using the modified ellipsoid formula (cube volume x ½) as below:
- Confirm the Tumor Volume does not exceed 10 cm3.
- Do not use STELFONTA if the tumor volume is >10 cm3.
STEP 2. Calculate the mL of STELFONTA to inject:
- Confirm the dose of STELFONTA does not exceed 0.25 mL/kg body weight and do not use if the calculated dose exceeds this.
- Do not exceed 5 mL per dog, regardless of tumor volume or body weight.
- The minimum dose of STELFONTA is 0.1 mL, regardless of tumor volume or body weight. If the calculated dose is <0.1 mL, administer 0.1 mL.
- Confirm the calculated dose of STELFONTA using the online calculator at www.stelfonta.com/calculator
(or scan the QR code to the right).
…
Warnings
(Additions and/or revision are underlined)
Human Safety Warnings
…
Animal Safety Warnings
…
Always administer the concomitant medications (prednisone or prednisolone, diphenhydramine, and famotidine), as directed in the Dosage and Administration section, with STELFONTA in order to decrease the potential for severe systemic adverse reactions, including death, from mast cell degranulation (see Adverse Reactions).
...
STELFONTA can induce a substantial local inflammatory reaction which may result in severe pain and swelling, bruising, cellulitis, extensive wound formation, and severe tissue sloughing extending away from the treated site. Consider administering analgesic medications prior to, during, and after treatment with STELFONTA in addition to the use of corticosteroids and both H1 and H2 receptor blocking agents.
Amputation of an extremity has been reported in some cases (see Post-Approval Experience).
Some dogs require wound care and pain management for an extended period.
…
Precautions
(Additions and/or revision are underlined)
…
Some discharge from the site following treatment is expected. Wear disposable gloves to clean the site with warm water as necessary.
After treatment with STELFONTA, dogs may require additional care of the treated site to aid in the healing process, especially if there is extensive wound formation (see Animal Safety Warnings and Post-Approval Experience).
Tongue lesions have been reported (see Post-Approval Experience). Do not allow the dog to lick the site for the first few days after treatment. Discourage excessive licking for the remainder of the healing period.
…
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2024)
The following adverse events are based on post-approval adverse drug experience reporting for STELFONTA. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs are listed in decreasing order of reporting frequency:
Injection site reactions (wound formation, swelling, pain, necrosis, skin sloughing, skin hemorrhage, bruising and erythema). These injection site reactions varied in severity and ranged from localized to extending away from the injection site.
Other signs reported include anorexia, lameness, lethargy, diarrhea, vomiting, fever, tachypnea/dyspnea, and ulceration or necrosis of tongue.
In some cases, when STELFONTA was used to treat a mast cell tumor on an extremity, the entire extremity became swollen, painful, and developed tissue sloughing. Some of these cases resulted in amputation.
In some cases, death (including euthanasia) has been reported as an outcome of the adverse events reported above.
Information for Dog Owners
(Additions and/or revision are underlined)
…
Owners should be advised to observe their dog for potential side effects, including signs of systemic mast cell degranulation, excessive pain and swelling, and excessive wound formation. Advise dog owners about the possibility of severe side effects, including amputation and death, when to contact a veterinarian, and how to care for the treated tumor site.
…
Discuss the importance of the concomitant medications and ensure that the owner is aware of the schedule of medications that should be administered. The owner should use the Medication Schedule on the CIS to keep track of the medications they have administered.
Discuss with owners that they should not allow the dog to lick the site for the first few days after treatment and they should discourage excessive licking for the remainder of the healing period.
An Elizabethan Collar may be utilized to prevent self-trauma of the treatment site. After treatment the owner may need to separate the dog from other household animals to prevent grooming and trauma to the treated site.
Client Information Sheet
(Additions and/or revisions are underlined)
This Client Information Sheet contains important information about STELFONTA. Before your dog is treated, you should carefully read this information and discuss the following with your veterinarian:
- How STELFONTA works.
- All parts of your dog’s treatment plan. It is very important to follow the treatment plan exactly as directed.
- The risk and benefits of STELFONTA, including the potential for serious side effects.
…
What additional medications need to be given to my dog before, on, and after the day of treatment with STELFONTA?
- To help prevent the potential for severe side effects that can occur, your veterinarian will prescribe three medications:
- You must start to give your dog the corticosteroid two days before the STELFONTA treatment day and continue for a total of 10 days.
- You will start giving your dog the H1 and H2 blockers on the STELFONTA treatment day and continue for a total of 8 days.
- Your veterinarian will fill out the medication schedule included in this Client Information Sheet for you to follow, so that you can give your dog the medications correctly.
- If you are unable to give your dog the medications as directed, talk to your veterinarian about other options. Do not skip these medications.
How will STELFONTA affect my dog?
…
- A wound will form where STELFONTA was administered. It is difficult to predict the size and severity of the wound formed. In some cases, an extensive wound that is deeper and/or larger than the original treatment site may develop, which may lead to unexpected complications.
- Tumors treated with STELFONTA typically go through a 4 to 6-week tumor breakdown and healing process.
… - During the tumor breakdown and healing process, your dog my require additional care of the treated tumor site to aid in the healing process.
…
Some dogs experience extensive wounds after STELFONTA treatment that take longer to heal, as in the case below.
What are some possible side effects of STELFONTA?
…
- In some cases, extensive swelling, severe pain, large amounts of discharge, odor, infection, or wound formation extending into the area surrounding the tumor site may develop, delaying wound healing and requiring additional management of the wound. If any of these occur, contact your veterinarian who will assess if your dog needs additional treatments during this time (e.g., pain medication, bandages, Elizabethan collar, antibiotics).
- Amputation has been reported as a result of extensive swelling and wound formation.
- Mast cell degranulation can occur, especially during the first 5-7 days after treatment. Mast cell degranulation is a type of allergic reaction that occurs when inflammatory substances are released from your dog’s tumor when mast cells are destroyed.
Mast cell degranulation can cause severe side effects and potentially be fatal. Contact your veterinarian if you notice any of the following signs:- trouble breathing or excessive panting
- excessive bruising and swelling
- vomiting
- diarrhea
- hives
- decreased appetite (refusal to eat for more than 1 day)
…
- Side effects can occur with or without warning, and in some cases may result in death.
Contact your veterinarian if you notice any of the following changes in your dog. These changes may mean your dog is experiencing a serious side effect.
...
- Excessive licking of the treatment site
- Tiredness, weakness, or refusal to eat for more than 1 day
… - Trouble breathing or excessive panting
- A rash or hives anywhere on the body
- Changes to the treated tumor site, including increased or excessive swelling and bruising, large amounts of discharge, strong odor, or extensive wound formation.
…
What do I need to know to safely care for my dog before and after treatment with STELFONTA (tigilanol tiglate injection)?
- Your veterinarian will prescribe medications to decrease the potential for allergic reactions due to mast cell degranulation that can occur during the treatment process. It is essential that you give the medications as prescribed (use the Medication Schedule below to help you keep track of the dosing schedule).
... - Notify your veterinarian if your dog seems uncomfortable. Your veterinarian may prescribe additional medications for pain.
… - Do not allow your dog to lick the tumor site for the first few days after treatment. Discourage excessive licking for the remainder of the healing period.
…
Use the Medication Schedule below to help you keep track of the medicine you need to give your dog.
Medication schedule
The following medications are to be given when your pet is treated with STELFONTA to help decrease and/or prevent side effects:
- Corticosteroid (prednisone or prednisolone): Start medication as directed two days before STELFONTA treatment day, continue on the treatment day, and for an additional 7 days after the treatment day, which will be a total of 10 days.
- H1 and H2 blockers (diphenhydramine and famotidine): Start medications as directed on STELFONTA treatment day and continue for an additional 7 days, which will be a total of 8 days.
- Use the following chart to help you keep track of the dosing schedule, make an ‘X’ in the box when that dose of medication is given. Do not administer the medication if the box is grayed out.
- If you are unable to give your dog the medications orally (by mouth), or your dog is vomiting or not eating, talk to your veterinarian to determine what other options may be used for administration. Do not skip these medications.
Note for veterinarian – the above chart is intended to be a visual aid for the client to administer the concomitant medications correctly. Fill in the chart with the specific dates of administration and the specific names of the medications prescribed.
Indications:
CREDELIO CAT kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) for one month in cats and kittens 8 weeks of age and older, and weighing 2.0 pounds or greater.
CREDELIO CAT is also indicated for the treatment and control of Ixodes scapularis (black-legged tick) infestations for one month in cats and kittens 6 months of age and older, and weighing 2.0 pounds or greater.
Summary of Changes:
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
The following safety-related changes were made to the labeling:
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2024):
The following adverse events are based on post-approval adverse drug experience reporting for CREDELIO CAT. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in cats are listed in decreasing order of reporting frequency:
Pruritus, vomiting, lethargy, behavioral changes (including hyperactivity, vocalization, and hiding), tachypnea, anorexia, muscle tremor, dyspnea, hyperthermia, and ataxia.
Indication:
GALLIPRANT (grapiprant tablets) is indicated for the control of pain and inflammation associated with osteoarthritis in dog.
Summary of Changes:
- Safety and risk information was updated in the Dosage and Administration, Warnings, Precautions, and Information for Dog Owners sections based on post-approval experience information for GALLIPRANT and to reflect that the safety profile is similar to other non-steroidal anti-inflammatory drugs approved for use in dogs.
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
- The Client Information Sheet (Information for Dog Owners) was updated to reflect the new safety and risk information and post-approval experience information.
The following safety-related changes were made to the labeling:
Dosage and Administration
(Additions and/or revisions are underlined)
Always provide “Information for Dog Owners” Sheet with prescription. Carefully consider the potential benefits and risks of GALLIPRANT and other treatment options before deciding to use GALLIPRANT. Use the lowest effective dose for the shortest duration consistent with individual response.
…
Warnings
(Additions and/or revisions are underlined)
…
All dogs should undergo a thorough history and physical examination before the initiation of NSAID therapy. Appropriate laboratory tests to establish hematological and serum biochemical baseline data prior to and periodically during administration of any NSAID is recommended.
Owners should be advised to observe for signs of potential drug toxicity (see Adverse Reactions, Post-Approval Experience, and Animal Safety) and be given an “Information for Dog Owners” Sheet about GALLIPRANT.
Do not administer GALLIPRANT in conjunction with any other oral or injectable NSAID or corticosteroid.
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Precautions
(Additions and/or revisions are underlined)
…
Adverse reactions in dogs receiving GALLIPRANT may include vomiting, diarrhea, decreased appetite, mucoid, watery, or bloody stools, and decreases in serum albumin and total protein (see Adverse Reactions and Post-Approval Experience).
If GALLIPRANT is used long term, appropriate monitoring is recommended.
GALLIPRANT is a non-cyclooxygenase (COX) inhibiting non-steroidal anti-inflammatory drug (NSAID). As a class, NSAIDs may be associated with gastrointestinal, renal, and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Dogs that have experienced adverse reactions from one NSAID may experience adverse reactions from another NSAID. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction.
Anesthetic drugs may affect renal perfusion; approach concomitant use of anesthetics and NSAIDs cautiously. Appropriate monitoring procedures (including ECG, blood pressure, and temperature regulation) should be employed during all surgical procedures. The use of parenteral fluids during surgery is recommended to decrease potential renal complications when using NSAIDs perioperatively.
If additional pain medication is needed after a daily dose of GALLIPRANT, a non-NSAID/non-corticosteroid class of analgesic may be necessary. Concurrent administration of potentially nephrotoxic drugs should be carefully approached and monitored. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Galliprant blocks the activity of the specific prostaglandin E2 (PGE2). Such anti-prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. NSAIDs possess the potential to produce gastrointestinal ulceration and/or gastrointestinal perforation. Do not use GALLIPRANT concomitantly with other anti-inflammatory drugs, such as NSAIDs or corticosteroids. Consider appropriate washout times when switching from GALLIPRANT to another NSAID (or vice versa) or when switching from corticosteroid use to GALLIPRANT use.
The concomitant use of protein-bound drugs with GALLIPRANT has not been studied in dogs. Commonly used protein-bound drugs include cardiac, anticonvulsant and behavioral medications. The influence of concomitant drugs that may inhibit metabolism of GALLIPRANT has not been evaluated. Drug compatibility should be monitored in patients requiring adjunctive therapy.
...
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2024)
The following adverse events are based on post-approval adverse drug experience reporting for GALLIPRANT. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in dogs are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
GI: diarrhea (with or without blood), vomiting (with or without blood), elevated pancreatic enzymes/pancreatitis, melena, bloody stool, hypoalbuminemia, abdominal pain, gastrointestinal ulcer.
General: anorexia, lethargy, weight loss, panting, hyperactivity.
Hepatic: elevated liver enzymes.
Renal/Urinary: increased BUN or creatinine, polydipsia, urinary incontinence/inappropriate urination, polyuria, renal failure.
Neurologic: ataxia, seizures.
Hematologic: anemia, thrombocytopenia.
Dermatologic/Immunologic: pruritus, allergic reactions (including facial edema and hives), immune mediated hemolytic anemia.
In some cases, death (including euthanasia) has been reported as an outcome of the adverse events listed above.
Information for Dog Owners
(Additions and/or revisions are underlined)
Owners should be advised of the potential for adverse reactions and be informed of the clinical signs associated with drug intolerance. Adverse reactions may include diarrhea, vomiting, decreased appetite, dark or tarry stools, increased water consumption, increased urination, pale gums due to anemia, jaundice (yellowing of gums, skin or white of the eye), lethargy, incoordination, seizures, and behavioral changes such as depression or restlessness. Serious adverse reactions associated with this drug class can occur without warning and, in some cases, result in death (see Adverse Reactions and Post-Approval Experience). Owners should be advised to discontinue GALLIPRANT therapy and contact their veterinarian immediately if signs of intolerance are observed. The vast majority of patients with drug related adverse reactions have recovered when the signs are recognized, the drug is withdrawn, and veterinary care, if appropriate, is initiated. Owners should be advised of the importance of periodic follow up for all dogs during administration of any NSAID.
Client Information Sheet (Information for Dog Owners)
(Additions and/or revisions are underlined)
…
What is GALLIPRANT?
GALLIPRANT is an EP4 prostaglandin receptor antagonist non-steroidal, anti-inflammatory drug (NSAID). As an anti-inflammatory, GALLIPRANT is indicated for the control of pain and inflammation associated with osteoarthritis in dogs.
Control of pain and inflammation may vary from dog to dog. Consult your veterinarian if your dog appears to be uncomfortable. GALLIPRANT may need to be given for an extended period of time. Use the lowest dose to provide adequate pain control. Always consult with your veterinarian before altering the dose.
…
Your dog should not take GALLIPRANT if:
- Your dog is presently taking aspirin, other NSAIDs (non-steroidal anti-inflammatory drugs such as carprofen or meloxicam) or corticosteroids (such as prednisone) unless directed by your veterinarian.
… - Your dog has bloody stool or vomit.
- Your dog has a pre-existing kidney or liver condition.
- Your dog has any condition predisposing to dehydration.
- Your dog is anorexic (loss of appetite).
…
GALLIPRANT should only be given to dogs. Do not use in cats.
People should not take GALLIPRANT. Keep GALLIPRANT and all medications out of reach of children. Consult a physician if you accidentally take GALLIPRANT.
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
Tell your veterinarian about:
- Any side effects your dog has experienced from GALLIPRANT or other NSAIDs. Dogs that have experienced side effects from one NSAID may experience adverse reactions from another NSAID.
… - If your dog has no appetite or a decreased appetite.
- Any heart, kidney, or liver disease your dog has had.
- Any other medical problems or allergies that your dog has now or has had in the past.
…
Talk to your veterinarian about:
- The signs of osteoarthritis you have observed (for example, limping or stiffness).
- What tests should be done prior to administering GALLIPRANT.
- How often your dog may need to be examined by your veterinarian.
- The risks and benefits of using GALLIPRANT.
What are the possible side effects that may occur in my dog during therapy with GALLIPRANT?
GALLIPRANT may cause some side effects in individual dogs. Serious side effects associated with this drug can occur with or without warning and, in some cases, result in death. The most common side effects associated with GALLIPRANT therapy involve the digestive tract (diarrhea, soft, mucoid stools, vomiting, and decreased appetite). Liver and kidney problems have also been reported.
Look for the following side effects that may indicate that your dog is having a problem with GALLIPRANT or may have another medical problem:
- Change in bowel movements such as diarrhea, soft mucoid stool, or change in stool color (black, tarry, or bloody stool).
- Vomiting.
- Decrease in appetite.
- Change in behavior, such as depression or restlessness.
- Incoordination or seizures.
- Change in drinking or urination.
- Yellowing of gums, skin, or whites of the eyes (jaundice).
It is important to stop the medication and contact your veterinarian immediately if you think your dog may have a medical problem or side effect while on GALLIPRANT.
If you have additional questions about possible side effects, talk with your veterinarian or call Elanco US Inc. at 1-888-545-5973. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
Can GALLIPRANT be given with other medications?
GALLIPRANT should not be given with aspirin, other non-steroidal anti-inflammatory drugs (such as carprofen or meloxicam) or corticosteroids (such as prednisone).
Tell your veterinarian about all medications that you have given your dog in the past, and any medications that you are planning to give with GALLIPRANT. This should include any medications that you can get without a prescription and any dietary supplements. Your veterinarian may want to evaluate the potential for any drug interactions and to assure drug compatibility.
What can I do in case my dog eats more than the prescribed amount of GALLIPRANT?
Contact your veterinarian immediately if your dog eats more than the prescribed amount of GALLIPRANT.
What else should I know about GALLIPRANT?
Keep GALLIPRANT in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
If you have any questions or concerns about GALLIPRANT or osteoarthritis, talk to your veterinarian. GALLIPRANT should only be given to the dog for which it was prescribed. It should be given to your dog only for the condition for which it was prescribed, at the prescribed dose, as directed by your veterinarian. Your veterinarian will determine if your dog is responding as expected and if your dog should continue receiving GALLIPRANT.
Indications:
Bravecto 1-Month kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations [Ixodes scapularis (black-legged tick), Dermacentor variabilis (American dog tick) and Rhipicephalus sanguineus (brown dog tick)] for one month in dogs and puppies 8 weeks of age and older and weighing 4.4 pounds or greater.
Bravecto 1-Month is also indicated for the treatment and control of Amblyomma americanum (lone star tick) infestations for one month in dogs and puppies 6 months of age and older and weighing 4.4 pounds or greater.
Summary of Changes:
- The Precautions section was revised to add language about adverse events being reported in breeding females following use of Bravecto (fluralaner) Chews.
- The Post-Approval Experience subsection of the Adverse Reactions section was updated, and new language was added regarding reproductive adverse events following use of Bravecto (fluralaner) Chews.
The following safety-related changes were made to the labeling:
Precautions
(Additions and/or revisions are underlined)
…
The safety of Bravecto 1-Month has not been evaluated in breeding, pregnant, and lactating dogs. Adverse events have been reported following use of Bravecto (fluralaner) Chews in breeding females.
Before use in breeding female dogs, refer to Post-Approval Experience and Animal Safety sections.
Adverse Reactions
(Updated Post-Approval Experience subsection, additions are underlined).
Post-Approval Experience (2024):
The following adverse events are based on post-approval adverse drug experience reporting for Bravecto 1-Month. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for dogs are listed in decreasing order of reporting frequency:
Vomiting, lethargy, diarrhea, anorexia, tremors, ataxia, seizure, pruritus, and allergic reactions (including hives, swelling, and erythema).
The following reproductive adverse events have been reported following use of Bravecto (fluralaner) Chews: birth defects (including limb deformities and cleft palate), stillbirth, and abortion.
Indications:
Synotic is indicated for the relief of pruritus and inflammation associated with acute and chronic otitis in the dog.
Summary of Changes:
- The Dosage and Administration section was updated to add a statement about reports of hearing loss with product use and instructions on how to proceed if hearing or vestibular dysfunction are noted during the course of treatment.
- The carton and bottle label were revised to add a pictogram, and the phrase “FOR OTIC USE IN DOGS ONLY” was revised to “FOR USE IN DOG EARS ONLY” to emphasize that the product is not for use in the eye.
The following safety-related changes were made to the labeling:
Dosage and Administration
(Additions and/or revision are underlined)
The recommended dose of SYNOTIC Otic Solution Veterinary is 4 to 6 drops (0.2 mL) per ear administered twice daily into the ear canal for a maximum period of 14 days. The total dosage used should not exceed 17 mL. It is recommended that the affected ear canal be cleansed by some appropriate method prior to the instillation of the solution. Following instillation, gentle external massage of the ear canal may aid in promoting an even distribution of the medication. Care should be taken to avoid contact of the medication with the dog’s eyes.
Contact of the bare hand with the medication should also be avoided.
Hearing loss has been reported in a small number of dogs after use. If hearing or vestibular dysfunction is noted during the course of treatment, discontinue use of Synotic immediately and flush the ear canal thoroughly with a nonototoxic solution.
Bottle Label
Indications:
RESFLOR GOLD is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis, and control of BRD-associated pyrexia in beef and non-lactating dairy cattle.
Summary of Changes:
- The Precautions section was revised to alert users to periparturient safety concerns in cows and heifers administered flunixin either before or after calving.
The following safety-related changes were made to the labeling:
Precautions
(Additions and/or revisions are underlined)
…
Not for use in animals intended for breeding purposes.
The effects of RESFLOR GOLD on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy. NSAIDs are known to have potential effects on both parturition and the estrous cycle. There may be a delay in the onset of estrus if flunixin is administered during the prostaglandin phase of the estrous cycle. Studies have associated the use of flunixin in cattle with a delay in parturition and prolonged labor (which may increase the risk of stillbirth), and interference with involution and expulsion of fetal membranes (which may increase the risk for placental retention and metritis).
…
Indications:
COMFORTIS kills fleas and is indicated for the prevention and treatment of flea infestations (Ctenocephalides felis), for one month, on cats and kittens 14 weeks of age and older and 4.1 pounds of body weight or greater.
Summary of Changes:
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
- The Client Information Sheet was updated to reflect the adverse events included in the Post-Approval Experience subsection.
The following safety-related changes were made to the labeling:
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2023)
The following adverse events are based on post-approval adverse drug experience reporting for COMFORTIS. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for cats are listed in decreasing order of reporting frequency:
Vomiting, depression/lethargy, anorexia, behavioral changes, pruritus, diarrhea, ataxia, hyperactivity, hypersalivation, panting, vocalization, dyspnea, twitching, and seizures.
In some cases, death has been reported. Some of these reports involved cats with cardiac disease, including cats with previously undiagnosed cardiac disease.
Following concomitant extra-label use of ivermectin with COMFORTIS, some cats have experienced the following clinical signs:
Ataxia, trembling/twitching, seizures, mydriasis, disorientation, and transient blindness.
Post-approval experience continues to support the safety of COMFORTIS when used concurrently with heartworm preventatives according to label directions.
Indications:
COMFORTIS kills fleas and is indicated for the prevention and treatment of flea infestations (Ctenocephalides felis), for one month, on dogs and puppies 14 weeks of age and older and 5.0 pounds of body weight or greater.
Summary of Changes:
- The Post-Approval Experience subsection of the Adverse Reactions section was updated.
- The Client Information Sheet was updated to reflect the new adverse events added to the Post-Approval Experience subsection.
The following safety-related changes were made to the labeling:
Adverse Reactions
(Updated Post-Approval Experience subsection, additions are underlined)
Post-Approval Experience (2023)
The following adverse events are based on post-approval adverse drug experience reporting for COMFORTIS. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported for dogs are listed in decreasing order of reporting frequency:
Vomiting, depression/lethargy, pruritus, anorexia, diarrhea, seizures, ataxia, trembling, behavioral changes, and hypersalivation.
Following concomitant extra-label use of ivermectin with COMFORTIS, some dogs have experienced the following clinical signs:
Trembling/twitching, salivation/drooling, seizures, ataxia, mydriasis, blindness, and disorientation.
Post-approval experience continues to support the safety of COMFORTIS when used concurrently with heartworm preventatives according to label directions.
Indications:
CREDELIO kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of tick infestations [Amblyomma americanum (lone star tick), Dermacentor variabilis (American dog tick), Ixodes scapularis (black-legged tick) and Rhipicephalus sanguineus (brown dog tick)] for one month in dogs and puppies 8 weeks of age and older, and weighing 4.4 pounds or greater.
Summary of Changes:
- A Post-Approval Experience subsection was added to the Adverse Reactions section.
The following safety-related changes were made to the labeling:
Adverse Reactions
(Newly added subsection)
Post-Approval Experience (2023)
The following adverse events are based on post-approval adverse drug experience reporting for CREDELIO. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events reported in dogs are listed in decreasing order of reporting frequency:
Vomiting, diarrhea (with and without blood), lethargy, anorexia, seizure, pruritus, ataxia, urinary-related signs (polyuria, polydipsia, urinary incontinence, and inappropriate urination), and muscle tremor.