FDA Releases Annual Summary of Sales and Distribution of Antimicrobials in 2024 for Use in Food-Producing Animals
Biomass-adjusted 2024 sales and distribution data also available
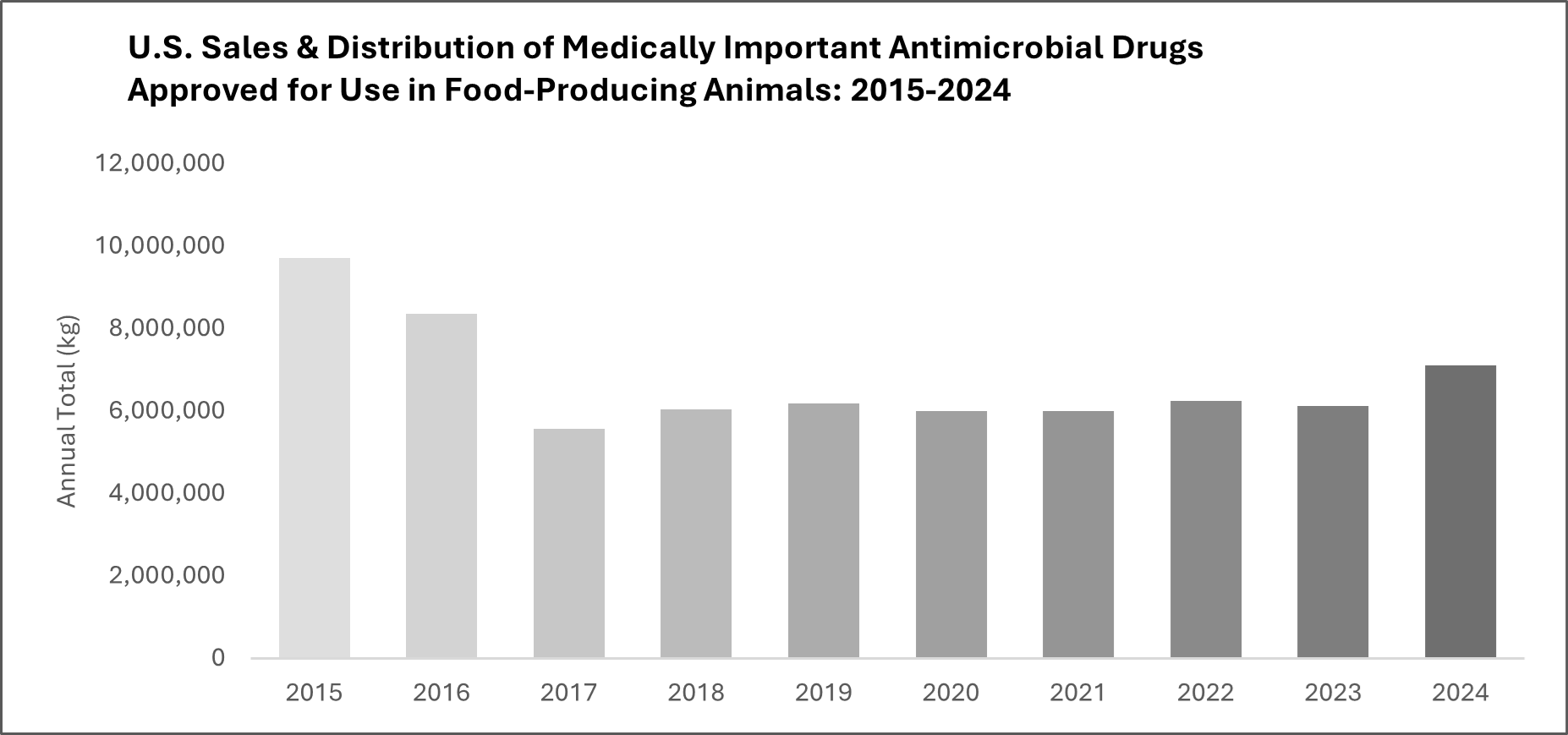
The U.S. Food and Drug Administration’s Center for Veterinary Medicine has published the 2024 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals, the most recent annual summary of data submitted to the agency by animal drug sponsors as required under the Federal Food, Drug, and Cosmetic Act. The 2024 data indicate that U.S. sales and distribution of medically important antimicrobial drugs approved for use in food-producing animals increased by 16 percent between 2023 and 2024. This increase is a departure from the generally stable annual sales and distribution data reported to FDA since 2017. To put these numbers in historical context, the overall sales volume in 2024 is 27 percent below peak sales in 2015.
Sales volume may fluctuate over time in response to various factors, including changing animal health needs, changes in animal populations, and changes in animal production practices. It is important to note that antimicrobial sales data do not necessarily reflect how much of the drugs are ultimately used in animals, only the volume that is sold.
The annual sales and distribution data are available in a dashboard reporting format that allows users to interact with the data and create data visualizations using criteria such as drug class, species, and year.
In addition, the FDA’s biomass-adjusted 2024 sales and distribution data is now available in the agency’s dashboard: Interactive Summary of Biomass-Adjusted Antimicrobial Sales Data. A biomass denominator adjusts annual antimicrobial sales data to account for the size of the population of a given livestock species in the U.S. potentially being treated with those drugs.
For more information about what readers should consider when analyzing the sales and distribution report, please review the FDA’s Questions and Answers: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals.
To learn about the FDA’s ongoing efforts to curb the development of antimicrobial resistance and foster antimicrobial stewardship in veterinary settings, see: Antimicrobial Stewardship in Veterinary Settings.
For more information
- 2024 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- Data Spreadsheet: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals (2015-2024)
- Biomass-Adjusted Antimicrobial Sales and Distribution Data in Food-Producing Animals: Interactive Summary
- Questions and Answers: Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals
- ADUFA Reports
- Final Rule to Collect Antimicrobial Sales and Distribution Information by Animal Species