Importation Program under Section 804 of the FD&C Act
FDA has developed a pathway under section 804 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) that allows importation of certain prescription drugs from Canada to:
- significantly reduce the cost of these drugs to the American consumer,
- without imposing additional risk to public health and safety.
FDA is committed to continuing to work with states and Indian tribes that seek to develop an importation proposal. States and Indian tribes may submit importation program proposals to FDA for review and authorization.
Section 804 Importation Program (SIP) Proposals
A SIP proposal needs to provide all the information required by the FD&C Act and FDA’s regulations. HHS provided information about demonstrating cost savings for the American consumer. A full list of requirements is provided in FDA’s regulations.
In particular, FDA regulations at 21 C.F.R. part 251 describe the requirements necessary for a sponsor of a SIP to demonstrate that their importation program will result in a significant reduction in the cost of eligible prescription drugs to the American consumer without posing any additional risk to the public’s health and safety. FDA has developed a resource, Tips for SIPs, which provides information to assist sponsors as they work to develop and implement a SIP proposal. A small entity compliance guide in question and answer format is also available to help in proposal development.
Visit responses to comments in the rulemaking for more information.
FDA Review Process
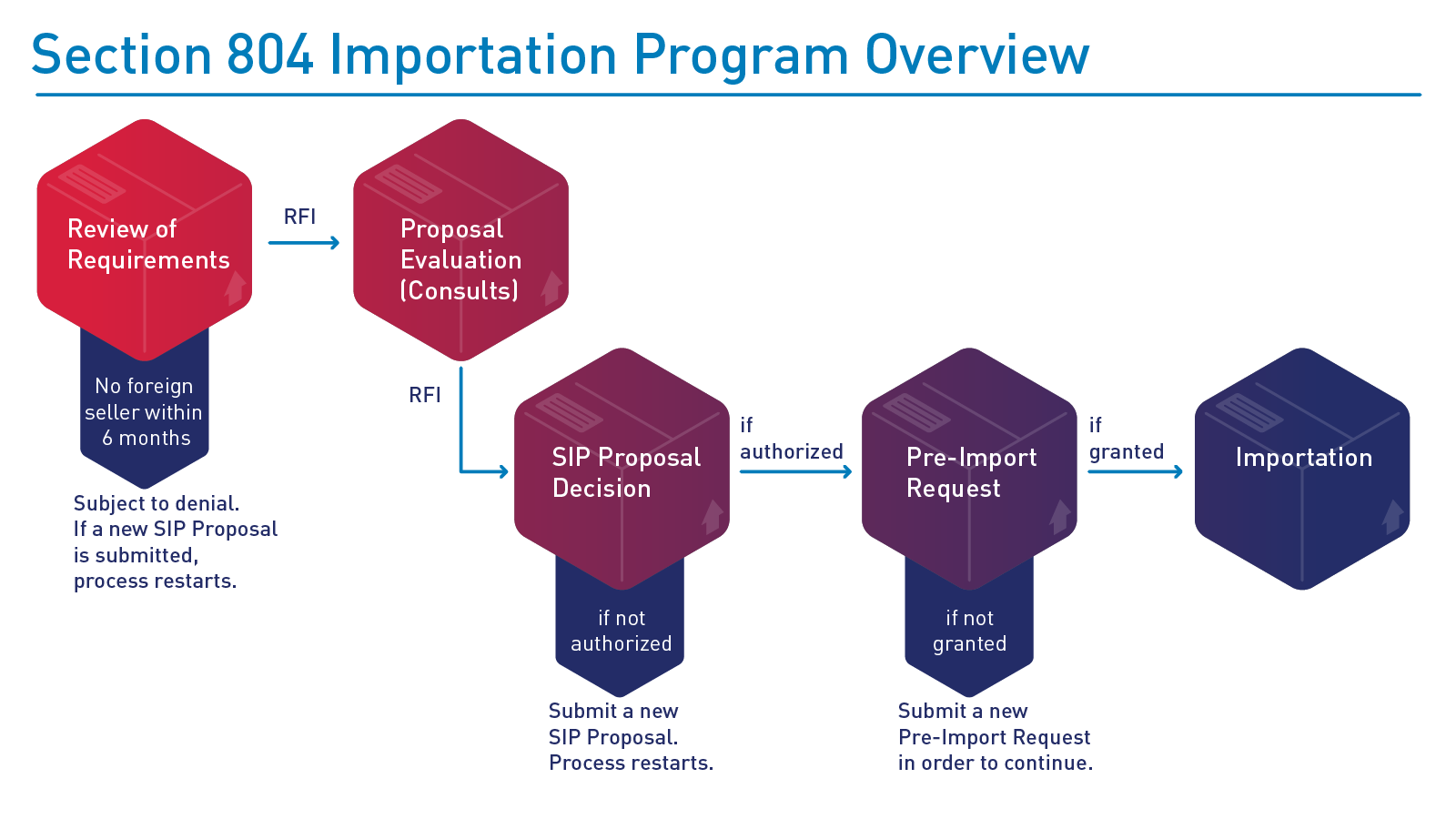
FDA’s evaluation of SIP proposals may include requests for additional information necessary to ensure the proposal meets the requirements in the statute and final rule. An example of FDA’s SIP proposal evaluation and potential implementation process is depicted below:
Policies and Actions
- FDA published Importation of Prescription Drugs Final Rule Questions and Answers; Small Entity Compliance Guide to help small entities better understand the final rule (May 2022)
- FDA met with representatives from several states, the National Academy for State Health Policy and HHS to discuss the development of SIP proposals (March 2022). Visit HHS presentation for more information: Projecting Cost Savings for the American Consumer (PDF - 195 KB)
- FDA issued a final rule, Importation of Prescription Drugs, which describes the requirements for SIPs and provides FDA responses to comments about the proposed rule (October 2020)
- Executive Order 14036 on Promoting Competition in the American Economy (July 2021)
FDA Authorization
- FDA Authorizes Florida’s Drug Importation Program
- FDA authorization letter to Florida’s Agency for Health Care Administration
Contact Us
In accordance with Executive Order 14036, FDA engages directly with states or Indian tribes who want to propose a program or would like more information about SIP proposals.
If you represent a state or Indian tribe, ask questions or submit a SIP proposal by emailing the FDA at: SIPDrugImportsandRFP@fda.hhs.gov.
States and Indian tribes interested in working with the agency on a SIP proposal can also contact FDA’s Intergovernmental Affairs Staff at IGA@fda.hhs.gov to begin the conversation.