AURA3: Nosebleeds
Project Patient Voice is intended to be used with a healthcare professional when discussing the potential symptoms related to a cancer and cancer treatment. Do not rely on Project Patient Voice alone to make decisions about medical care. Do not use Project Patient Voice to substitute for advice from your health care professional. Conclusions about patient experiences with symptoms may be limited because not all symptoms may have been captured by the patient-reported questionnaire.
Download symptom data (XLSX, 24KB)
In AURA3 Study, Patients Were Asked: "In the last 7 days, how OFTEN did you have NOSEBLEEDS?"
Patients scored the frequency of their Nosebleeds on a 5-point scale (Never, Rarely, Occasionally, Frequently, Almost Constantly)
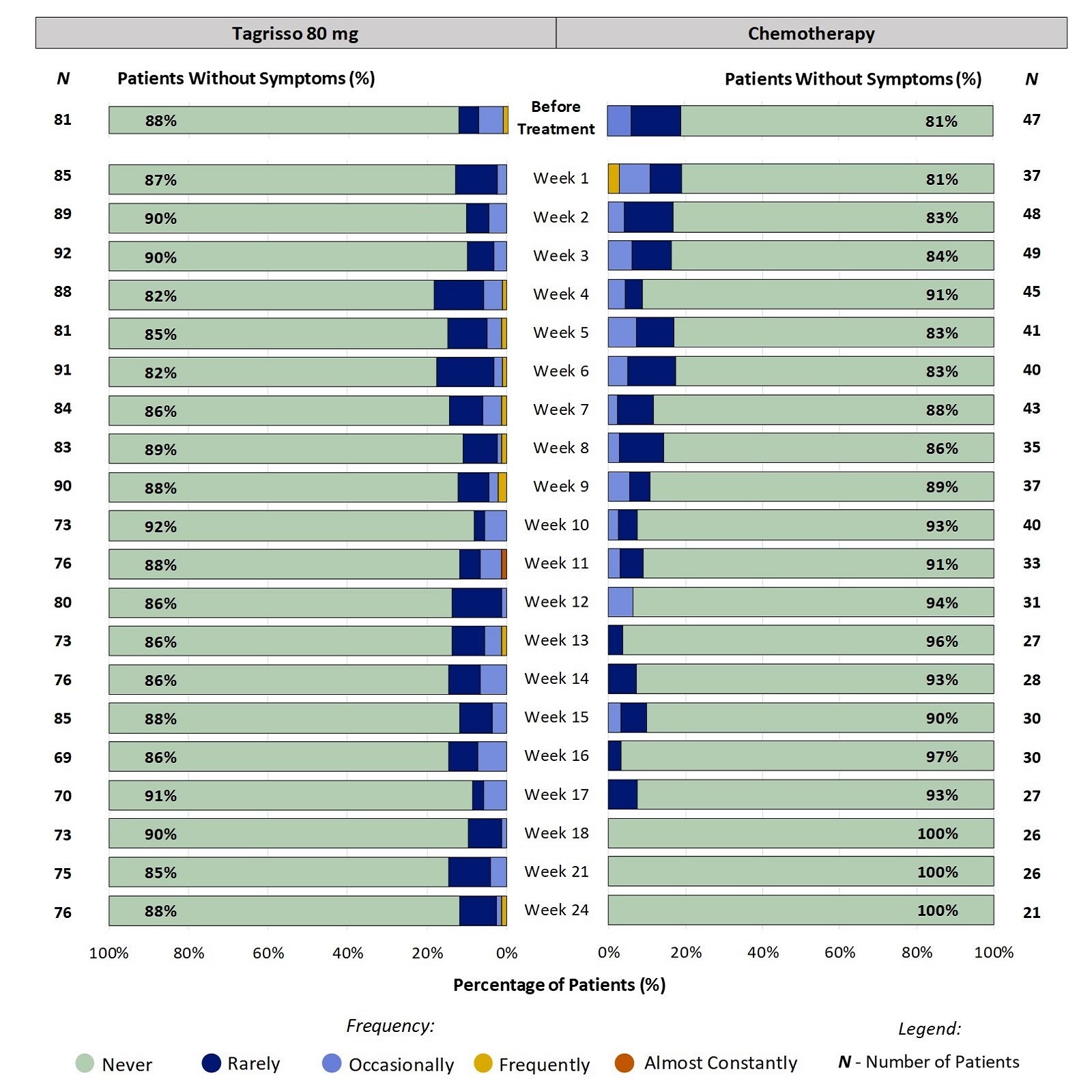
Patient-Reported Nosebleeds During the First 24 Weeks on Treatment for Patients Who Completed a Questionnaire:
Figure 1 shows the percentage of patients reporting how often they had Nosebleeds at each time point. For example, at week 2, 10% of patients taking Tagrisso reported Nosebleeds (ranging from Rarely to Occasionally). The range of patients who had any Nosebleeds during the first 24 weeks of treatment with Tagrisso was between 8% - 18%. Click here for more information on how to read the graphs below.
Figure 1. Patient-Reported Nosebleeds During the First 24 Weeks on Treatment
All responses from patients' experiences just before and up to week 24 on-treatment were included in the analysis. Some patients did not report their symptoms every week, therefore the number of patients may vary between weeks. Furthermore, not all patients remained on the treatment for 24 weeks (e.g., some stop treatment for worsening disease) which is a reason for the change in the number of patients over the course of treatment.
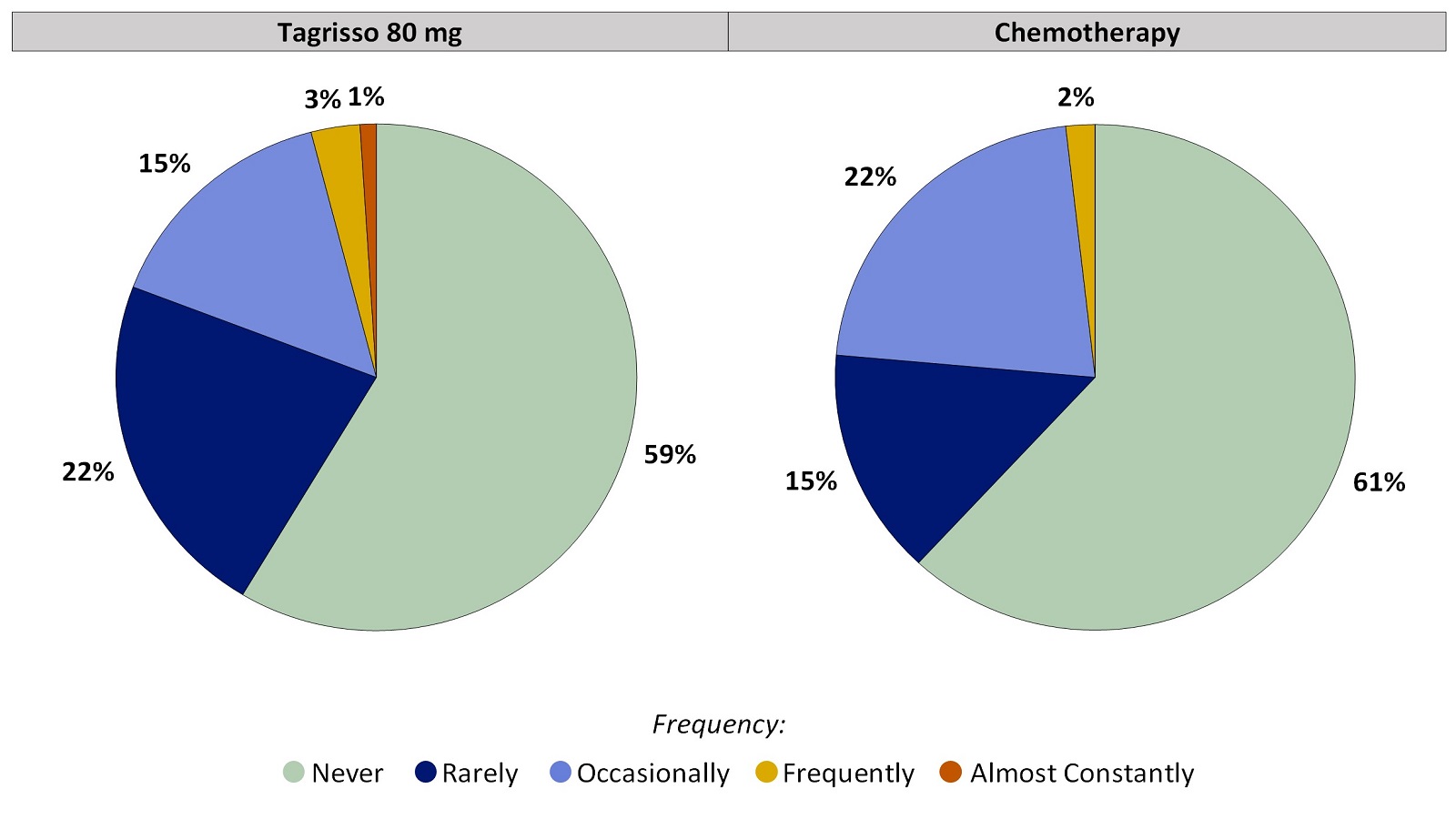
Worst Response Option for Nosebleeds That Patients Reported During the First 24 Weeks on Treatment
Figure 2. Worst Patient-Reported Nosebleeds During the First 24 Weeks on Treatment
Patients with at least one on-treatment Nosebleeds score were included in the analysis. Tagrisso (N=99), Chemotherapy (N=55).
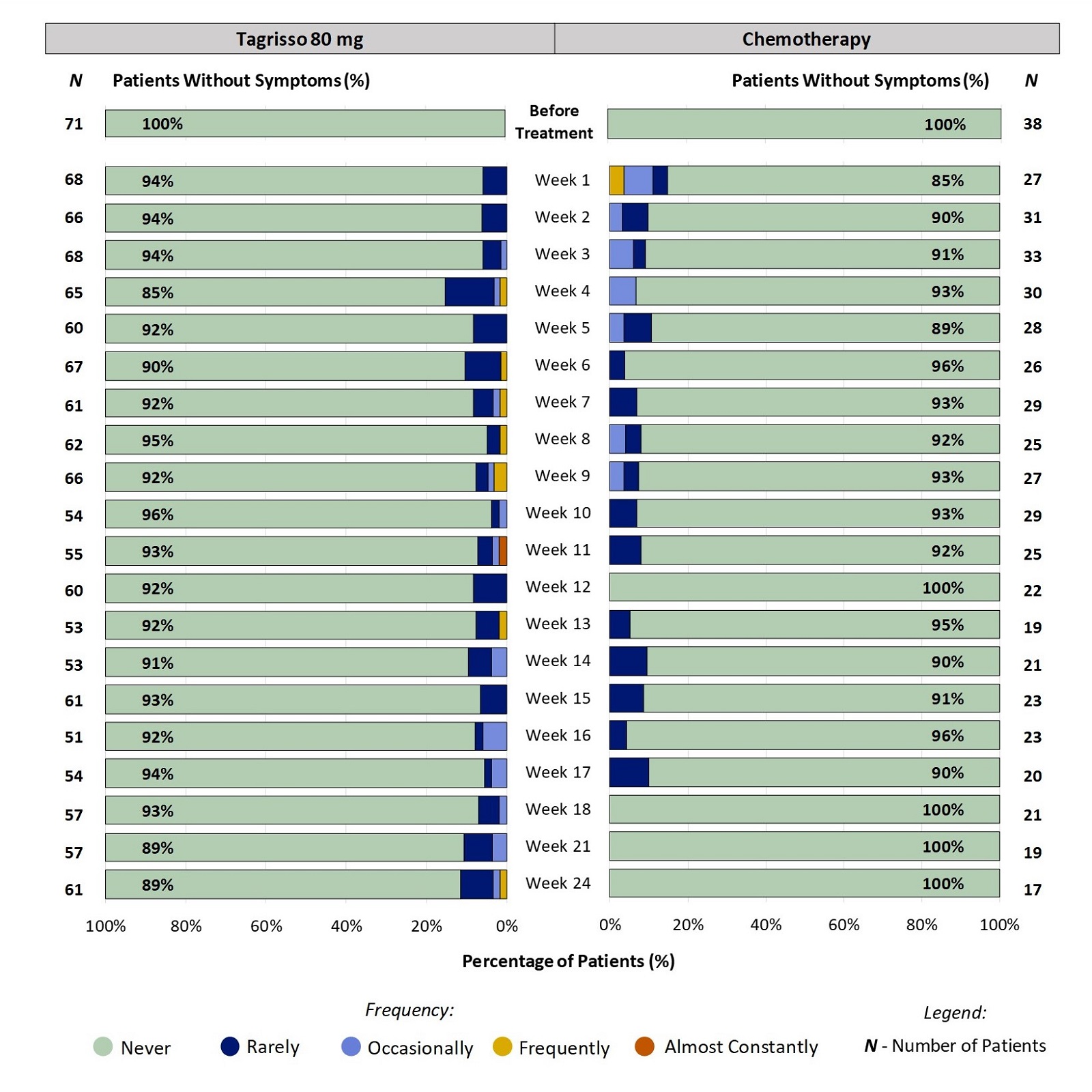
Some Patients Did Not Report Nosebleeds Before Treatment:
For patients that did not report Nosebleeds before treatment, Figure 3 shows the percentage of patients reporting how often they had Nosebleeds between weeks 1 and 24.
Figure 3. Patient-Reported Nosebleeds During the First 24 Weeks on Treatment: Patients Without Nosebleeds Before Treatment
All responses from patients who did not report Nosebleeds before treatment were included in the analysis. Some patients did not report their symptoms every week, therefore the number of patients may vary between weeks. Furthermore, not all patients remained on the treatment for 24 weeks (e.g., some stop treatment for worsening disease) which is a reason for the change in the number of patients over the course of treatment.
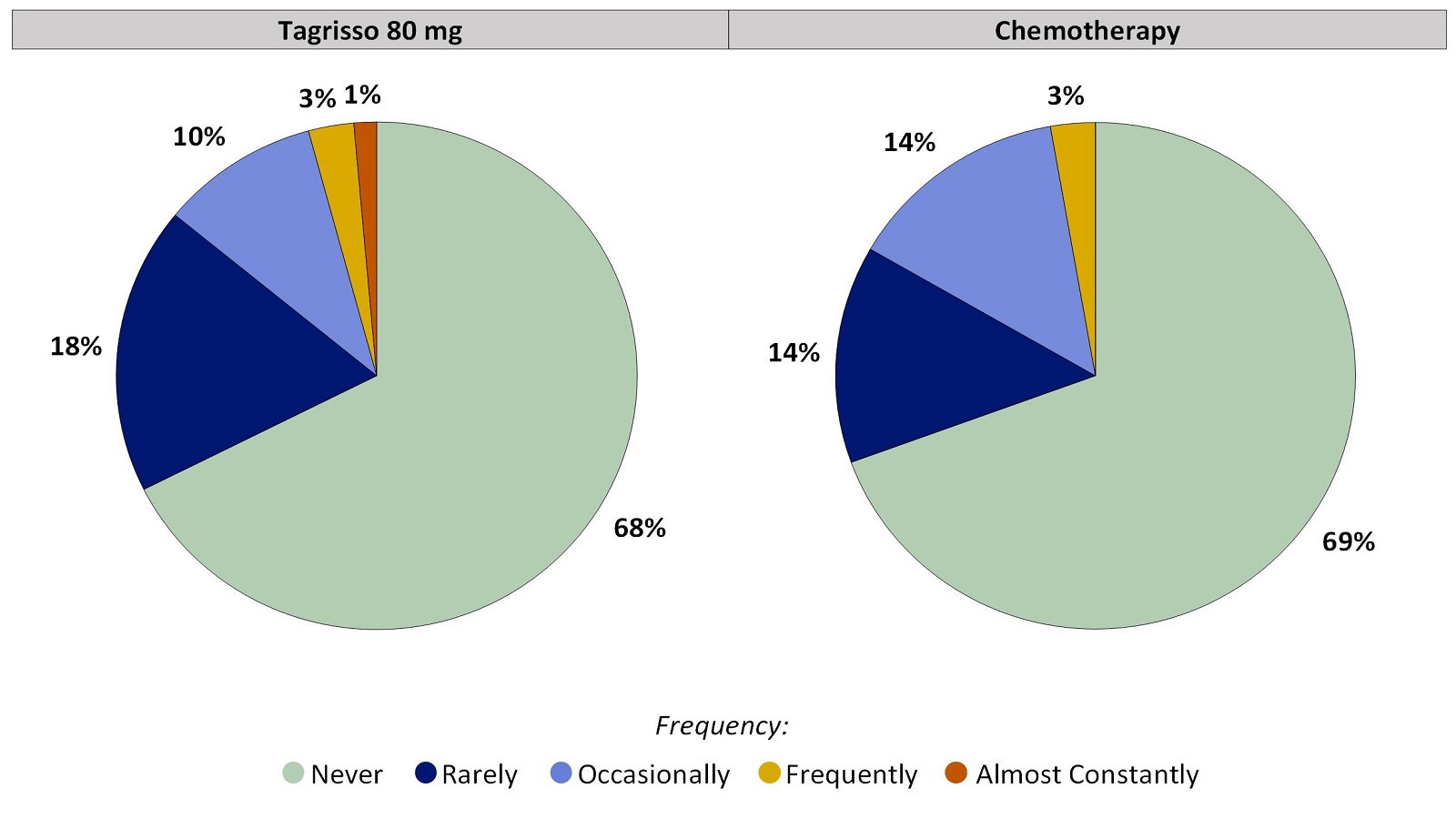
Worst Response Option for Nosebleeds That Patients Reported During the First 24 Weeks on Treatment, for Patients Who Did Not Have Nosebleeds Before Treatment:
Figure 4. Worst Patient-Reported Nosebleeds During the First 24 Weeks on Treatment: Patients Without Nosebleeds Before Treatment
Patients who had no Nosebleeds before treatment and at least one on-treatment Nosebleeds score were included in the analysis. Tagrisso (N=71), Chemotherapy (N=36).