COMPANY ANNOUNCEMENT
AurobindoPharma USA, Inc. Initiates a Voluntary Nationwide Consumer Level Recall Expansion of 38 Lots of Amlodipine Valsartan Tablets USP and Valsartan Tablets, USP due to the detection of NDEA (N-Nitrosodiethylamine) Impurity.
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
Prescription Drugs - Reason for Announcement:
-
Recall Reason DescriptionDue to the detection of NDEA (N-Nitrosodiethylamine) Impurity
- Company Name:
- AurobindoPharma USA, Inc.
- Brand Name:
-
Brand Name(s)Aurobindo, Acetris
- Product Description:
-
Product DescriptionValsartan and Amlodipine and Valsartan tablets
Company Announcement
AurobindoPharma USA, Inc. is conducting a voluntary recall expansion of 38 lots of Valsartan and Amlodipine and Valsartan tablets to the consumer level due to the detection of trace amounts of an unexpected impurity found in the finished drug producAurobindoPharma USA, Inc. is conducting a voluntary recall expansion of 38 lots of Valsartan and Amlodipine and Valsartan tablets to the consumer level due to the detection of trace amounts of an unexpected impurity found in the finished drug product. This recall is an expansion of the recall initiated 12/31/18 The impurity detected in the finished drug product is N-nitrosodiethylamine (NDEA), which is a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, and has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC) classification. The expansion relates to lots distributed under the labels for AurobindoPharma USA, Inc. and Acetris Health, LLC. To date, AurobindoPharma USA, Inc. has not received any reports of adverse events related to this recall.
Amlodipine Valsartan Tablets USP and Valsartan Tablets USP are indicated to control high blood pressure and for the treatment of heart failure. Patients who prescribed Amlodipine Valsartan Tablets USP and Valsartan Tablets USP should continue taking their medication, as the risk of harm to the patient’s health may be higher if the treatment is stopped immediately without any alternative treatment. Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication.
The products subject to recall are listed below and packaged in bottles. The product can be identified by checking the product name, manufacturer details and batch or lot number on the bottle containing these products.
| NDC | Name and strength | Count | Lot number | Expiry |
|---|---|---|---|---|
|
ACETRIS LOTS |
||||
| 52343-122-30 | Valsartan Tablets USP 40mg | 30 | 470170038A | 19-Oct |
| 52343-122-30 | Valsartan Tablets USP 40mg | 30 | 470180010A | 20-Feb |
| 52343-122-30 | Valsartan Tablets USP 40mg | 30 | 470180012A | 20-Mar |
| 52343-123-90 | Valsartan Tablets USP 80mg | 90 | 471170019A | 19-Oct |
| 52343-123-90 | Valsartan Tablets USP 80mg | 90 | 471180006A | 20-Mar |
| 52343-123-90 | Valsartan Tablets USP 80mg | 90 | 471180007A | 20-Mar |
| 52343-123-90 | Valsartan Tablets USP 80mg | 90 | 471180016A | 20-May |
| 52343-124-90 | Valsartan Tablets USP 160mg | 90 | 472180005B | 20-Feb |
| 52343-124-90 | Valsartan Tablets USP 160mg | 90 | 472180011A | 20-Apr |
| 52343-124-90 | Valsartan Tablets USP 160mg | 90 | 472180012A | 20-Apr |
| 52343-125-90 | Valsartan Tablets USP 320mg | 90 | 473180007A | 20-Mar |
| 52343-125-90 | Valsartan Tablets USP 320mg | 90 | 473180008A | 20-Mar |
| 52343-125-90 | Valsartan Tablets USP 320mg | 90 | 473180011A | 20-Apr |
| 52343-125-90 | Valsartan Tablets, USP 320mg | 90 | 473180020B1 | 20-Jul |
|
AUROBINDO LOTS |
||||
| 65862-570-30 | Valsartan Tablets USP 40mg | 30 | 470180008A | 20-Feb |
| 65862-570-30 | Valsartan Tablets USP 40mg | 30 | 470180014A | 20-Mar |
| 65862-570-30 | Valsartan Tablets USP 40mg | 30 | 470180016A | 20-Mar |
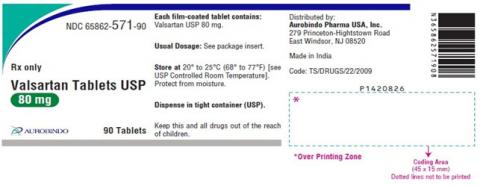
| 65862-571-90 | Valsartan Tablets USP 80mg | 90 | 471170015A | 19-Sep |
| 65862-571-90 | Valsartan Tablets USP 80mg | 90 | 471180004A | 20-Feb |
| 65862-571-90 | Valsartan Tablets USP 80mg | 90 | 471180005A | 20-Feb |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180001A | 20-Jan |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180002A | 20-Jan |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180003A | 20-Jan |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180004A | 20-Jan |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180007A | 20-Mar |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180008A | 20-Mar |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180009A | 20-Mar |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180010A | 20-Mar |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180013A | 20-Apr |
| 65862-572-90 | Valsartan Tablets USP 160mg | 90 | 472180014A | 20-Apr |
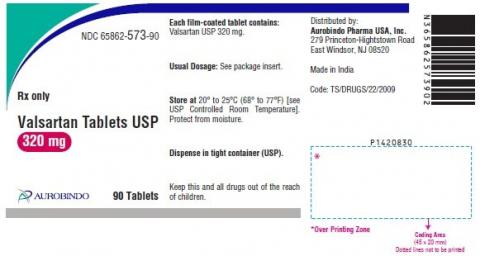
| 65862-573-90 | Valsartan Tablets USP 320mg | 90 | 473180004A | 20-Feb |
| 65862-573-90 | Valsartan Tablets USP 320mg | 90 | 473180005A | 20-Feb |
| 65862-573-90 | Valsartan Tablets USP 320mg | 90 | 473180006A | 20-Mar |
| 65862-573-90 | Valsartan Tablets USP 320mg | 90 | 473180017A | 20-May |
| 65862-739-30 | Amlodipine and Valsartan Tablets USP10mg/160mg | 30 | VFSA17007-A | 19-Oct |
| 65862-570-30 | Valsartan Tablets, USP 40mg | 30 | 470180032A | 20-May |
| 65862-573-90 | Valsartan Tablets, USP 320mg | 90 | 473170019A | 19-Oct |
| 65862-573-90 | Valsartan Tablets, USP 320mg | 90 | 473180016A | 20-May |
Amlodipine Valsartan Tablets USP and Valsartan Tablets USP were distributed nationwide to AurobindoPharma USA, Inc. and Aceteris Health LLC wholesale, distributor, repackager and retail customers. AurobindoPharma USA, Inc. is notifying its distributors and customers by phone and in writing to immediately discontinue distribution of the specific lots being recalled and to notify their sub-accounts. AurobindoPharma USA, Inc. is arranging for return of all recalled products to Inmar. Instructions for returning recalled products are given in the recall letter.
Consumers with medical questions regarding this recall or to report an adverse event can contact AurobindoPharma USA, Inc. at:
- 1-866-850-2876 Option 2
- pvg@aurobindousa.com
Consumers should also contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.
Any general questions regarding the return of this product please contact Inmar\CLS-Medturn at 1-877-208-2407 or email aurobindorecalls@inmar.com (live calls received 9 am -5:00 pm Eastern Time).
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm Call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
- Consumers:
- AurobindoPharma USA, Inc.
- 1-866-850-2876 Option 2
- pvg@aurobindousa.com
- Media:
- Inmar\CLS-Medturn
- 1-877-208-2407
- aurobindorecalls@inmar.com