COMPANY ANNOUNCEMENT
Prinston Pharmaceutical Inc Issues Voluntary Nationwide Recall of Valsartan and Valsartan HCTZ Tablets Due to Detection of a Trace Amount of Unexpected Impurity, N-Nitrosodimethylamine (NDMA) in The Products
When a company announces a recall, market withdrawal, or safety alert, the FDA posts the company's announcement as a public service. FDA does not endorse either the product or the company.
Read Announcement View Product PhotosSummary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Drugs
- Reason for Announcement:
-
Recall Reason DescriptionDue to Detection of a Trace Amount of Unexpected Impurity, N-Nitrosodimethylamine (NDMA)
- Company Name:
- Prinston Pharmaceutical Inc. dba Solco Healthcare LLC
- Brand Name:
-
Brand Name(s)Prinston Pharmaceutical Inc. dba Solco Healthcare LLC
- Product Description:
-
Product DescriptionValsartan Tablets, 40 mg, 80mg, 160mg, and 320mg; and Valsartan-Hydrochlorothiazide Tablets, 80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg, and 320mg/25mg
Company Announcement
FOR IMMEDIATE RELEASE - CRANBURY, NEW JERSEY - Prinston Pharmaceutical Inc., dba Solco Healthcare LLC., is voluntarily recalling all lots of Valsartan Tablets, 40 mg, 80mg, 160mg, and 320mg; and Valsartan-Hydrochlorothiazide Tablets, 80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg, and 320mg/25mg to the consumer level. This product recall is due to the detection of a trace amount of an unexpected impurity, N-nitrosodimethylamine (NDMA), in an active pharmaceutical ingredient by the manufacturer – Zhejiang Huahai Pharmaceutical Co. Ltd. -- in the manufacture of the subject product lots. This impurity has been classified as a probable human carcinogen as per International Agency for Research on Cancer (IARC) classification. However, at present Prinston is unaware of any evidence that NDMA has resulted in any harm to patients taking the drugs subject to this recall. To date, Prinston Pharmaceutical Inc. has not received any reports of adverse events related to this recall.
The products are indicated for the treatment of hypertension.
Consumers and Patients should contact their pharmacist or physician who can advise them about an alternative treatment prior to returning their medication. Patients who are on valsartan should continue taking their medication, as the risk of harm to a patient's health may be higher if the treatment is stopped immediately without any alternative treatment.
| Product | NDC Code | Lot Number | Expiry Dates | Distribution Date |
|---|---|---|---|---|
| VALSARTAN TABLETS 40MG 30CT | 43547-367-03 | All lots | From Jul 18 to Jan 20 | Oct 2015 – Jun 2018 |
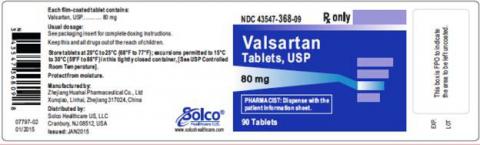
| VALSARTAN TABLETS 80MG 90CT | 43547-368-09 | All lots | From Jul 18 to Jan 20 | Oct 2015 – Jun 2018 |
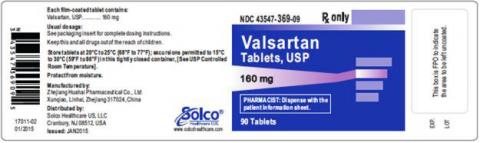
| VALSARTAN TABLETS 160MG 90CT | 43547-369-09 | All lots | From Jul 18 to Jan 20 | Oct 2015 – Jun 2018 |
| VALSARTAN TABLETS 320MG 90CT | 43547-370-09 | All lots | From Jul 18 to Jan 20 | Oct 2015 – Jun 2018 |
| VALSARTAN/HCTZ 80MG/12.5MG 90CT TABLETS | 43547-311-09 | All lots | From Jul 18 to Jan 20 | Jun 2016 – Jun 2018 |
| VALSARTAN/HCTZ 160MG/12.5MG 90CT TABLETS | 43547-312-09 | All lots | From Jul 18 to Jan 20 | Jun 2016 – Jun 2018 |
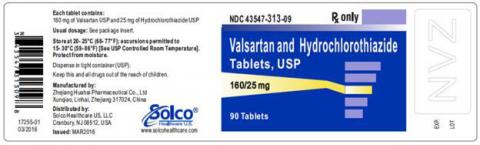
| VALSARTAN/HCTZ 160MG/25MG 90CT TABLETS | 43547-313-09 | All lots | From Jul 18 to Jan 20 | Jun 2016 – Jun 2018 |
| VALSARTAN/HCTZ 320MG/12.5MG 90CT TABLETS | 43547-314-09 | All lots | From Jul 18 to Jan 20 | Jun 2016 – Jun 2018 |
| VALSARTAN/HCTZ 320MG/25MG 90CT TABLETS | 43547-315-09 | All lots | From Jul 18 to Jan 20 | Jun 2016 – Jun 2018 |
The lot number and expiry date information can be found on the manufacturer's unit (see photographs below of packaged product bottle labels).
Retail pharmacies in possession of any unused products: Valsartan Tablets, 40 mg, 80mg, 160mg, and 320mg; and Valsartan-HCTZ Tablets, 80mg/12.5mg, 160mg/12.5mg, 160mg/25mg, 320mg/12.5mg, and 320mg/25mg, within expiry dates from Jul 2018 to Jan 2020 should immediately return the product by following the instructions below:
- Pharmacists and wholesalers are asked to check their inventories, segregate any impacted inventory.
- Immediate discontinue use and distribution of the identified lot numbers. A credit memo will be issued covering the quantity of your product returned.
- Return products to:
DLSS (Dohmen Life Science Services)
Attn: Returns Department
4580 S. Mendenhall, Memphis, TN 38141
Note: A call tag, a pre-printed, pre-paid return label will be provided to you for product return; return is free of charge. For call tag, please contact Solco Customer Service at 1-866-931-9829, Option 5, Monday through Friday (9am to 5pm EST). Wholesalers: No call is necessary, just send debit memo via email or fax to: customerservice@solcohealthcare.com; fax 1-866-931-0709.
Solco is notifying its distributors and customers by letter and email and is arranging for return of all recalled products. Pharmacies and wholesalers that received the impacted products will receive a letter as well as a copy of this press release with their recall notification information. Additional information regarding this recall's affected product lots and expiry dates can be found at http://www.solcohealthcare.com/uploads/news/ValsartanHCTZRecallAffactedLots.pdf or to download at http://www.solcohealthcare.com/uploads/news/ValsartanHCTZRecallAffactedLots.xlsx.
Consumers and patients with medical-related questions regarding this recall, please contact Solco at 888-679-5120 between the hours of 9:00 a.m. to 5:00 p.m. EST Monday through Friday. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to using this product.
Adverse reactions or quality problems associated with the use of this product may be reported to FDA's MedWatch Adverse Event Reporting program either by phone, on line, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178.
This Product Recall is being made with the knowledge of the United States Food and Drug Administration (FDA).
Pharmacies, Distributors, Wholesalers
customerservice@solcohealthcare.com1-866-931-9829, Option 5
1-866-931-0709, for Product Return
Company Contact Information
- Consumers:
- 888-679-5120