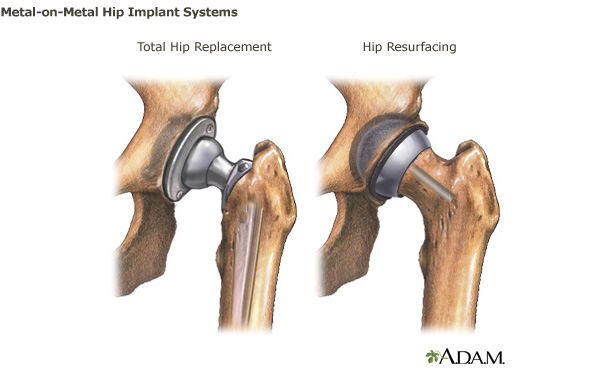
Metal-on-Metal Hip Implants
Hip joint deterioration can lead to pain, stiffness or difficulty walking. When these symptoms do not respond to conservative treatment, such as physical therapy, patients may be advised to undergo total hip replacement or hip resurfacing. There are several types of hip implants including total hip replacement, hip resurfacing, hemi-arthroplasty, bi-polar, and dual mobility systems.
This webpage provides information on hip systems in which the “ball and socket” of the device are both made from metal. which include metal-on-metal articulation and how metal-on-metal implants differ from other hip implants and recommendations for patients and health care providers about the benefits and risks of these products. The information provided on this webpage is not meant to replace a discussion with your health care provider.
In January 2013, the FDA published a proposed order to allow for notice and comment regarding the FDA's recommendation to change the requirements for all metal-on-metal (MoM) total hip implants from premarket notification, to premarket approval, the most stringent regulatory category of the FDA's oversight for medical devices. A final order was published in February 2016 and the requirement for filing premarket approvals was effective in May 2016. Since that time, all manufacturers of MoM total hip implants are required to stop marketing their devices and submit premarket approvals that must be approved before the devices can be marketed. Premarket approval is based on a determination by the FDA that the application contains sufficient valid scientific evidence to reasonably assure that the device is safe and effective for its intended use, and requires manufacturers to apply for the FDA's approval before making changes to the device, its labeling or manufacturing, and imposes certain other annual reporting requirements.
To date, there are no FDA-approved metal-on-metal total hip replacement devices marketed for use in the US. There are two FDA-approved metal-on-metal hip resurfacing devices available.
Some patients who had a hip replacement prior to May 18,2016 may have received a metal-on-metal hip implant. The FDA has provided Information for Patients with a metal-on-metal hip implant. If you are unsure whether you have a metal-on-metal hip implant, or if you have questions, talk to your doctor.