Good Manufacturing Practices for the 21st Century for Food Processing (2004 Study) Section 2: Literature Review of Common Food Safety Problems and Applicable Controls
August 9, 2004
This section presents ERG's literature review of preventive controls for microbiological, chemical, and physical food safety problems in the food processing industry. Microbiological safety hazards cause most of the foodborne illnesses and include pathogenic bacteria, viruses, and parasites. Historically, pathogenic bacteria have been the most prevalent food safety hazard, with viral cases following closely behind according to the most recent CDC report on the etiology of foodborne illness (CDC, 2004). Chemical food safety hazards vary widely, but the most common problems cited in the literature include contamination with pesticides, allergens, and natural toxins, including scrombotoxins found in fish and mycotoxins found in crops. Foreign objects, or physical safety hazards, are the least likely to affect large numbers of people and usually are easily recognized.
Many of the microbiological food safety problems discussed in the literature can potentially be addressed by good manufacturing practices (GMPs) codified in 21 CFR 110, such as proper employee hygiene, adequate training, and effective cleaning and sanitizing of the manufacturing equipment and environment. For example, niche environments, which are sites within the manufacturing environment that can harbor bacteria, are a significant cause of post-processing contamination but difficult to reach with average cleaning and sanitizing procedures. Food plants that put in a greater than average effort must identify and eliminate niches by taking apart equipment in order to minimize the risk of post-processing contamination from niche environments. Others take an even more stringent approach by applying a post-package pasteurization method, virtually eliminating the risk of post-processing contamination due to niche environments.
Many chemical food safety problems are also addressed by following good manufacturing practices, such as pest control and proper storage. The rigor of the controls in place varies by plant, however. Further, some food safety problems, such as allergen control, may be better addressed by a Hazard Analysis and Critical Control Point (HACCP) plan in addition to GMPs. Physical hazards may also be better controlled by a HACCP plan. Controls may include foreign body detection systems, such as metal detectors, in addition to putting preventive measures in place.
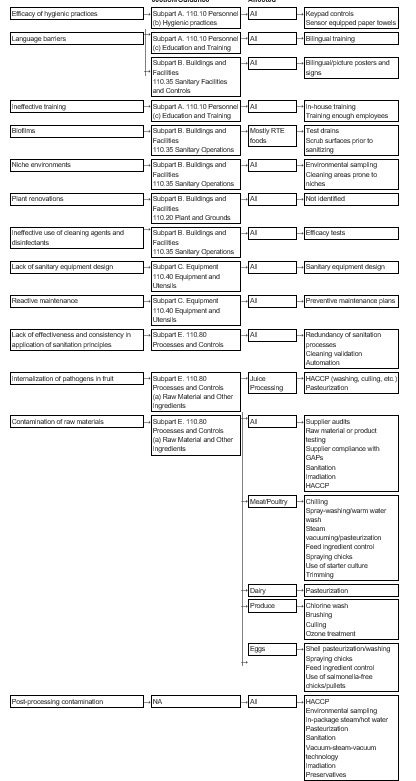
Table 2-1 summarizes the range of problems associated with each type of hazard as identified in the literature. The following three sections provide a more detailed overview of each hazard and the preventive controls to address each problem, as noted in the literature. Each section also includes a summary flowchart that highlights the potential problems, the relevant CFR section or guidance that addresses each problem, the industry/product covered, and the types of preventive controls typically recommended to eliminate or minimize the type of food safety hazard risk posed. Finally, Section 2.4 discusses other issues to consider when evaluating food safety controls, in addition to GMPs.
2.1 Microbiological Safety
The microbiological safety hazards include pathogenic bacteria, viruses, and parasites. Some of the problems that lead to the contamination of food with these microorganisms at the processor level can be easily remedied with improved employee training programs and effective hygienic practices. Others are more difficult to control, such as post-processing contamination with Listeria monocytogenes, a pathogen that is ubiquitous in the processing environment.
Inefficient hygienic practices among employees. Employee hygiene is paramount to plant sanitation and is one of the leading causes of food contamination (Higgins, 2002). One of the challenges that food processors have to overcome is how to motivate employees to comply with hygienic practices. Training is one step in the process, but is often not enough to ensure employee compliance. Companies have adopted several aids to ensure employee compliance. For example, Atlanta's Buckhead Beef Company requires workers to key in their Social Security Numbers to activate the hand sanitizer dispensers on the plant floor. The company then uses the collected data to impose financial reprisals on employees found to be deficient in hand-sanitizing practices. Other controls include a sensor-equipped towel that prevents the cross-contamination that can occur with hand cranks. These units also count the number of towels dispensed. A signal dispenser that beeps when users have washed their hands sufficiently is also available to ensure adequate hand-washing time.
Language barriers. Current training programs, even those that include Spanish signage and instructional manuals, can be inadequate if the first language of plant employees is one other than English or Spanish. Even Spanish training materials can be problematic due to dialectical differences in translations. Some industry experts therefore recommend a picture-and-symbol approach to training to overcome language barriers (Higgins, 2002).
Table 2-1. Range of Processor-Level Problems by Type of Food Safety Hazard Posed
| Microbiological Safety | Inefficient employee hygiene practices |
|---|---|
| Language barriers | |
| Ineffective training of employees | |
| Biofilms | |
| Niche environments | |
| Plant renovations | |
| Ineffective use of cleaning agents/disinfectants | |
| Lack of sanitary equipment design | |
| Reactive instead of routine maintenance | |
| Ineffective application of sanitation principles | |
| Internalization of pathogens in fruit | |
| Contamination of raw materials | |
| Post-processing contamination | |
| Chemical Safety | Raw material contamination with pesticides |
| Indiscriminate spraying of facilities against pests | |
| Mistaken identity of pesticides | |
| Spillage of pesticides | |
| Adding too much of an approved ingredient | |
| Raw material contamination with an allergen | |
| In-line cross-contamination with an allergen | |
| Contamination by utilization of rework | |
| Cross-contamination from maintenance tools | |
| Cross-contamination from conveyor belts | |
| Incorrect labeling or packaging | |
| Older equipment (more difficult to clean) | |
| Raw material contamination with natural toxins | |
| Mycotoxin infestation due to drought | |
| Mycotoxin infestation due to insect damage | |
| Mycotoxin infestation due to delayed harvesting | |
| Mycotoxin infestation due to mechanical damage | |
| Mycotoxin infestation due to moisture/heat | |
| Patulin production in apples | |
| Corrosion of metal containers/equipment/utensils | |
| Contamination with cleaner/sanitizer residue | |
| Adding too much of an approved ingredient | |
| Physical Safety | Foreign matter in raw materials |
| Poorly maintained equipment/lines | |
| Light fixture breakage | |
| Foreign matter introduction during storage |
Ineffective training of employees. Although effective training is crucial to ensuring that sanitation standards are met, it is not clear that current training methods are sufficient. In the third Annual Best Manufacturing Practices Survey conducted by the Food Engineering magazine in 2002, a panel of food manufacturing professionals rated employee training as the lowest among all food safety measures in terms of effectiveness (Gregerson, 2002). Employee training that companies conduct may be too generic. For example, external consultants may not be familiar enough with a plant's operations and requirements to give effective advice. Other impediments to effective training might include training the wrong people, not training enough people, or not providing enough training (Blackburn and McClure, 2002).
Biofilms. Biofilms occur when bacteria form a slime layer upon a surface and provide an environment for pathogens to proliferate. The adhesion of pathogenic bacteria to a biofilm is a food safety hazard because the biofilm can detach and become a significant source of food contamination. Cleaning to remove biofilms prior to sanitation is often sufficient to prevent this problem. However, studies have shown that attached bacteria may survive conventional cleaning methods (Austin and Berferon, as cited in Stopforth et al, 2002). Adequate cleaning prior to sanitizing is therefore paramount to controlling this problem. Further, coating drains and equipment parts with antimicrobial material can counteract biofilms although it does not eliminate the need for proper cleaning and sanitizing (Higgins, 2003).
Niche environments. Niche environments are sites within the manufacturing environment where bacteria can get established, multiply, and contaminate the food processed. These sites may be impossible to reach and clean with normal cleaning and sanitizing procedures. Examples include hollow rollers on conveyors, cracked tubular support rods, the space between close-fitting metal-to-metal or metal-to-plastic parts, worn or cracked rubber seals around doors, and on-off valves and switches (Tompkin, 2002). Tompkin (2002) provides an extensive list of potential niches. Manufacturers must identify and eliminate niches. Microbiological sampling of the environment and equipment can detect a niche. Third-party validation of test results might be useful to further establish confidence in environmental sampling results. Further, sanitary equipment design can help prevent niches (AMI, 2003). Proper maintenance to keep equipment parts from providing potential niches is also essential.
Plant renovations. Outbreaks of listeriosis have been linked to environmental contamination of food caused by plant renovations (FDA/CFSAN, 2001a). While no data were identified in the literature on this issue, plant renovations are likely to require revisions in standard operating procedures (SOPs) to prevent contamination due to changes in processes.
Ineffective use of cleaning agents and disinfectants. Different cleaning agents vary in their ability to remove different soil types (Blackburn and McClure, 2002). Thus, the correct choice of cleaning agent is essential to ensure effective cleaning in a food processing facility. The efficacy of disinfectants is dependent on microbial species, pH, presence of biofilms, temperature, concentration, and contact time (Stopforth et al., 2002; Blackburn and McClure, 2002). Stopforth et al. (2002) found that commonly used disinfectants were not as effective as desired, possibly due to inadequate pre-cleaning steps. While there were no examples in the literature of plants having problems with this issue, the potential for ineffective sanitation is clearly present. Food manufacturers should always confirm the efficacy of their cleaning and disinfection programs with tests from the supplying companies or in-house trials (Blackburn and McClure, 2002).
Lack of sanitary equipment design. Good hygienic design of equipment prevents or minimizes microbiological contamination of food. The materials used for food processing equipment should be easily cleanable. As noted earlier, niche environments are known sources of pathogens; surfaces also deteriorate with age, and this abrasion makes cleaning more difficult (Blackburn and McClure, 2002). For cleaning and sanitation to be effective, all parts of the equipment should be readily accessible. Another way to improve equipment hygiene is to use antimicrobial coatings on equipment parts (Higgins, 2003).
Reactive rather than routine/predictive maintenance. In the Best Manufacturing Practices Survey conducted by Food Engineering magazine in 2001, 56 percent of respondents reported having routine preventive programs (Gregerson, 2002). Only 8.5 percent of respondents noted having predictive maintenance programs; the remaining respondents described their programs as reactive in nature, i.e., "run it 'til it breaks." Reactive maintenance can result in food contamination before a failure is identified. Niches can develop or controls can become defective in processing equipment that is not routinely maintained. For example, in 1994, a Listeria monocytogenes outbreak was linked to the use of defective processing equipment in the production of chocolate milk (FDA/CFSAN, 2001a).
Ineffective application of sanitation principles. It may be difficult for a food processor to apply sanitation principles consistently and effectively to each batch of product. Food processors have found that improving the effectiveness of sanitation principles is dependent on using redundant processing controls (FDA/CFSAN, 1999c). Validation of cleaning processes may also be necessary. Automation that makes it unnecessary for humans to conduct the cleaning, such as robotic spray washers, may also improve sanitation. The extent to which these practices are used in the industry is unclear and should be explored with industry experts.
Internalization of pathogens in fruit. Fruit is usually contaminated by direct or indirect contact with animal feces. Studies have shown that pathogens can infiltrate fruit through damaged or decayed areas or through the flower end of the fruit (FDA/CFSAN, 1999a; FDA/CFSAN, 1999b; FDA/CFSAN, 1999c). While employing best control practices--such as not using dropped fruit, removing damaged fruit, and washing/brushing fruit prior to processing--minimizes these risks, the problem can only be controlled with some certainty by a kill step, such as pasteurization. Other possible controls are listed in the FDA Report of 1997 Inspections of Fresh, Unpasteurized Apple Cider Manufacturers and listed again in the annotated bibliography.
Contamination of raw materials. Many pathogens, like E. coli and Salmonella, enter the food processing environment via raw materials contaminated with those pathogens. A number of studies have shown that methods currently in place to prevent this are not sufficient (FDA/CFSAN, 1999a;
FDA/CFSAN, 1999b; FDA/CFSAN, 1999c; Riordan et al., 2001; Tilden et al., 2002). Raw material contamination can affect any industry, but is more common in industries that use animal-derived products or products at risk of cross-contamination by animal feces. There are numerous preventive controls available to address the hazard. Some controls minimize the risks of raw material contamination (i.e., ensuring that raw material suppliers comply with good agricultural practices) and others (i.e., irradiation, pasteurization) involve a kill-step to eliminate any pathogens.
Post-processing contamination. Products can also be contaminated if the post-processing environment, utensils, or equipment have been contaminated with a pathogen. This issue is especially relevant to the pathogen Listeria monocytogenes, due to its hardiness and pervasiveness in the environment. Effective controls against post-process contamination include eliminating the pathogen from the post-processing environment by using environmental sampling to eliminate niches, effective sanitation, and various in-package pasteurization methods. Use of preservatives, such as nisin, to slow down the growth of Listeria monocytogene are also becoming more common.
Figure 2-1: Microbiological Safety Problems, Related CFR Section or Guidance, Industries Affected, and Sample Preventive Controls Suggested
2.2 Chemical Safety
Chemical safety hazards include intentionally added chemicals (e.g., allergens), unintentionally added chemicals (e.g., cleaners and solvents), and natural toxins (e.g., mycotoxins). Chemicals can also contaminate food through corrosion of metal processing equipment/utensils and residues of cleaning chemicals left on processing equipment. Further, adding too much of an approved ingredient, such as a vitamin in vitamin-fortified products, may compromise the safety of foods.
Raw material contamination with pesticides. FDA has found that roughly 1 percent of sampled domestic produce has pesticide residue in violation of EPA standards (FDA/CFSAN, 2002). While the incidence of contamination is low, consumers remain concerned about pesticide residues. Aside from washing and testing the produce, manufacturers can select produce from organic suppliers to avoid raw material contaminated with pesticides. Other alternative farming systems, such as low-input sustainable agriculture (LISA) and integrated pest management, are also control options at the farm level (Moulton, 1992). These systems, which use much less pesticide than conventional agricultural systems, rely on biological, chemical, cultural, and physical principles and tools to control pests throughout the farming operation. Other preventive control options may include genetic engineering with resistance against pests or developing safer chemicals (Moulton, 1992).
Indiscriminate spraying of facilities against pests. Chemicals can contaminate food if pesticides against insects and rodents are used indiscriminately in a processing facility. Therefore, food experts generally recommend that pest control be performed only by professionals to avoid residues in food (Folks, 2001).
Mistaken identity of pesticides. Food can become contaminated with pesticides if pesticide container labels are misread or when products are stored in containers that have had another use. The best way to control the risk of mistaken identity is to store pesticides away from food ingredients, keep an inventory of pesticides, and store the products in their original containers (Tybor, 1990; Folks, 2001; Bryan, 1997).
Spillage of pesticides or other chemicals. Pesticides should be handled like poisons to avoid potential spillage. Storing chemicals away from food and packaging materials will minimize accidental spillage of pesticides and other chemicals (Tybor, 1990). Further, processors should only use food-grade lubricants and greases in manufacturing.
Corrosion of metal containers/equipment/utensils. Metal poisoning can occur when heavy metals leach into food from equipment, containers, or utensils. When highly acidic foods (e.g., citrus fruits, fruit drinks, fruit pie fillings, tomato products, sauerkraut, or carbonated beverages) come into contact with potentially corrosive materials, the metals can leach into the food (Tybor, 1990). One solution to the problem is to use appropriate, non-corrosive materials in food processing.
Residue from cleaning and sanitizing. If equipment and other food handling materials are not rinsed well, then residue from detergents, cleaning compounds, drain cleaners, polishers, and sanitizers can contaminate a food product. This problem can best be controlled by properly training personnel about cleaning and sanitizing (Folks, 2001; Tybor, 1990).
Accidentally adding too much of an approved ingredient. Some substances, such as preservatives, nutritional additives, color additives, and flavor enhancers, are intentionally added to food products. But adding an approved ingredient in inordinate amounts by accident--such as adding too much nitrite to cured meat--can result in a toxic product (Bryan et al., 1997). Thus, Tybor (1990) recommends that nitrite be stored in a locked cabinet and weighed and bagged separately before being added to any product. Nutritional safety issues can also arise when product labels' nutrition information is incorrect. Thus, it can be dangerous to public health when too little or too much of a specified nutrient is added. For example, malnutrition can occur if infant formula does not deliver the expected nutrient content during its shelf life. Due to the risk involved, infant formula quality control procedures and labeling requirements are addressed outside of GMPs in 21 CFR 106 and 107, respectively. There are also many examples of nutritional food safety issues arising when too much of a nutrient gets added to a product unintentionally. For example, some vitamins that are added to fortified foods (such as Vitamin A) are known to be toxic at high doses. And iron, a necessary dietary component, can cause severe illness and death if too much is ingested. Controlling chemicals by keeping an inventory of additives minimizes the occurrence of this type of contamination (Folks, 2001).
Natural toxins. Food can be contaminated with naturally occurring chemicals that cause disease. Toxins such as mycotoxins (discussed further below) and marine toxins are naturally produced under certain conditions. Given that these toxins generally occur in raw materials, especially crops and seafood, manufacturers should require suppliers to certify hat the products they purchase are free from natural toxins.
Cross-contamination with allergens on production lines. A product can become cross- contaminated with allergens on the production line. To minimize the risk of cross-contamination, equipment must be cleaned and sanitized to remove all traces of allergens when the next run includes product that should not contain allergens (Minnesota Department of Agriculture, 2003). Wash-down techniques may need adjustment to ensure that they remove allergens as well as pathogens (Higgins, 2000). Rinsing with water only or only cleaning at the end of the day is not adequate (FDA/CFSAN, 2001a). Some equipment may need to be disassembled to be cleaned. The cleaning process should be verified by visual inspection. Enzyme-linked immunosorbent assay (ELISA) tests can also help verify cleaning procedures (Deibel et al., 1997; Morris, 2002). Manufacturers may choose to physically separate lines for allergen- and nonallergen-containing products (Morris, 2002). This may be too costly for most plants; scheduling longer production runs to minimize changeovers, with allergen-containing product runs scheduled at the end of the day, may be a more suitable alternative (Deibel et al., 1997; FDA/CFSAN, 2001b; Floyd, 2000; Gregerson, 2003; Minnesota Department of Agriculture, 2003; Morris, 2002). Crossover points on production lines, including conveyor belts that transport products, should be enclosed to prevent cross-contamination. Physical detachments and lockouts can be used for equipment common to allergen- and nonallergen-containing foods (Deibel et al., 1997). Maintenance tools should be color-coded to prevent cross-contamination (FDA/CFSAN, 2001b; Morris, 2002). Allergenic materials should be stored separately from nonallergenic materials, with dedicated utensils and containers. Putting all of the ingredients for a specific batch on a pallet before taking them to the processing area, or "staging," will also minimize the risk of cross-contamination. Line clearance, such as removing all the ingredients from the production area and checking for cleanliness, can also help prevent cross-contamination (Floyd, 2000). Product can also be tested for the presence of allergens, although this does not appear to be a common industry practice (FDA/CFSAN, 2001a). Finally, allergens should be evaluated as part of a hazard analysis, and a HACCP plan or similar approach can be taken to identify process areas that are at high risk for contamination with allergens (Morris, 2002).
Raw material contamination with allergens. When controlling a production process for allergens, manufacturers must maintain a close working relationship with suppliers of raw materials. The ingredient specification should provide assurance that the product is allergen free (Deibel et al., 1997; FDA/CFSAN, 2001c). Manufacturers should also obtain full ingredient lists from their suppliers (Deibel et al., 1997; Gregerson, 2003). Reconditioned ingredients and oils should not be purchased (Minnesota Department of Agriculture, 2003). The manufacturer should also audit suppliers each year to determine other products that are run on the same production line, whether any allergenic processing aids or rework have been used in the product, and whether any contamination from other common equipment could have occurred (Gregerson, 2003). A training program may be necessary to educate suppliers about allergen control, especially if suppliers have not implemented an allergen control plan (Deibel et al., 1997, Minnesota Department of Agriculture, 2003).
Contamination with allergens by utilization of rework. Proper use of rework is essential to prevent contamination of product with allergens. A documented rework plan should be available. Rework areas, equipment, and containers must be clearly identified and documented, as well as the rework itself (Deibel et al., 1997; Gregerson, 2003). This can be done through the use of color tags, plastic liners, or bar coding.
Not declaring an allergen on labeling. Unavoidable product contamination with allergens may occur if it is impossible to verify that all residue has been removed from a line or if other controls cannot be put in place (Floyd, 2000). A good manufacturing practice includes reviewing the labeling to ensure that the allergen is declared. However, a study of inspections conducted by FDA/ CFSAN (2001a) indicated that many firms do not have label review policies. Further, a large percentage of these manufacturers had undeclared allergens in their products. Controls to prevent this problem can include removing old label and packaging inventories from plants, verifying labels by scanning bar codes, and conducting label audits (FDA/CFSAN, 2001b; FDA/CFSAN, 2001c; Minnesota Department of Agriculture, 2003).
Older equipment. Effective cleaning is paramount to controlling allergen contamination. Older equipment, however, may not be designed to verify cleaning with a visual inspection (Deibel et al., 1997). As noted in the section on microbiological issues and controls, all parts of the equipment should be readily accessible and visible for cleaning and sanitation to be effective. Further, equipment surfaces should not harbor allergens. Gregerson (2003) reports one such case in which cross-contamination with allergens occurred due to the surface nicks on the processing table. Thus, sanitary equipment design is necessary to ensure proper removal of allergens from equipment.
Infestation of mycotoxins due to drought. Toxigenic fungi, or mycotoxins, are found primarily in foods of plant origin, although they can also pass through the food chain in milk and meat. Drought can encourage the growth of mycotoxins in certain crops. For example, drought stress can cause aflatoxin, a type of mycotoxin, to grow in corn and treenuts (Moss, 2002). Drought can be minimized through adequate irrigation schedules (Park et al., 1999). Thermal and chemical treatments are also available for use on crop that is already affected by mycotoxins (Park et al., 1999). Thermal inactivation, however, is not effective on certain types of mycotoxins, such as aflatoxin. Chemical treatments, such as ammoniation and activated carbons and clays, are other possible controls (Boutrif, 1999; Horne et al., 1989; Park et al., 1999; Suttajit, 1989).
Infestation of mycotoxins due to damage. Insect damage is associated with high levels of mycotoxin infection, as is mechanical damage from harvesters (Boutrif, 1999; Moss, 2002; Park et al., 1999). Diseases, such as ear rot in corn, also cause damage that leaves the crop susceptible to mycotoxin infestation (Moss, 2002). Delayed harvesting can also make crops more susceptible to disease due to higher moisture levels (Park et al., 1999). Damage to the product, whether through insect feeding or mechanical harvesters, provides a potential entry point for the mold that produces the mycotoxin. Controls available include pest management to prevent insect damage, breeding cultivars that are resistant to pest damage, timely harvesting, hand picking or electronic sorting to remove damaged crops, and thermal or chemical treatment as noted above (Boutrif, 1999; Moss, 2002; Park et al., 1999; Suttajit, 1989). Possible biological control of insects and diseases in the field is also being investigated (Moss, 2002).
Infestation of mycotoxins due to moisture/heat during storage. Post-harvest storage that protects the product from heat and moisture is essential to prevent mycotoxin infestation (Boutrif, 1999). Grains should be dried as soon as feasible, and storage under modified atmospheric conditions is desirable (GASCA/CTA, 1997). Products should be dried rapidly to less than 10 percent moisture (Park et al., 1999). Products can also be sampled for mycotoxins during storage (Boutrif, 1999). Methods include visual inspection with black light, ELISA tests, and complex laboratory analysis using high-pressure liquid chromatography (Horne et al., 1989). While prevention with proper storage conditions is the best way to control mycotoxin infestation, thermal and chemical inactivation, as described earlier, can control any mycotoxins that do form under storage.
Patulin production in apples. Patulin is a mycotoxin that is produced by a number of molds associated with fruit spoilage (Bisessur et al., 2001). Control methods often used in the production of apple juice include using tree-picked apples, culling apples, washing apples, charcoal treatment, chemical preservation using sulfur dioxide, gamma radiation, fermentation, trimming of fungus-infected apples, and clarification methods (Bisessur et al., 2001; Jackson, et al., 2003).
2.3 Physical Safety
Materials that do not belong in food, like glass or metal, cause physical safety hazards. A physical safety hazard is any extraneous object or foreign matter in food that can cause injury or illness in the person consuming the product (Folks, 2001). Rocks, metal, wood, and other objects are sometimes found in raw ingredients. Further, contamination can occur during transport, processing, and distribution of foods due to equipment failure, accidents, or negligence (Institute of Medicine/National Research Council, 1998). Separation equipment should be used to separate the foreign bodies from the product. Detection methods include metal detectors, x-ray machines, and optical systems (Wallin and Haycock, 1998).
Foreign matter in raw materials. Sources of foreign matter in raw materials can include nails from pallets and boxes, ingested metal from animals, harvesting machinery parts, elements from the field, veterinary instruments, caps, lids, closures, and more (Wallin and Haycock, 1998). Mechanical harvesters will often collect more than the product. Processors can include separation equipment, such as destoners, air cleaners, magnets, screens, sieves, traps, scalpers, and washers as part of their production lines. For example, grain processors use four screens to remove foreign materials (Stier, 2001). Foreign matter in raw materials can be controlled with raw material inspections and vendor certifications or guarantees from suppliers. X-ray technology is also available to examine incoming material (Folks, 2001).
Poorly maintained equipment and lines. Pieces of equipment can break off and enter food products during processing if equipment is poorly maintained. Routine or preventive maintenance and other periodic checks of equipment can minimize the risk from this safety issue. Risk is further minimized with the use of metal detectors and x-ray machinery. Proper calibration of equipment and minimizing contact between pieces of machinery is also helpful (Folks, 2001; Stier, 2001).
Lighting fixture/other glass breakage. Glass can be controlled by having a glass breakage policy, such as throwing away all food and containers within 10 feet of the incident (Stier, 2001). Light fixtures can be protected so that if they break, the glass does not spill out (Folks, 2001). Other controls include examining of empty glass containers visually or cleaning a container with water or compressed air and inverting the container to remove any shards. Capping equipment should be properly calibrated and lines should be monitored for evidence of glass breakage. X-ray technology can also be helpful in identifying glass pieces in food (Olson, 2002).
Human factors. Production line workers can be a major source of contamination. For example, jewelry can fall off or break, fingernails can break, and pens can fall into food. Jewelry removal is required under GMPs. If pens are metallic, a metal detector can detect them. Production workers' fingernails should be cut short and gloves should be worn under certain processing conditions.
Introduction of foreign matter during storage. Pests can enter products during storage, leaving remnants behind. Effective pest control is the solution. It can include preventive measures such as filling in all non-functional openings in a building; fully sealing doors, windows, and vents; protecting intake points with filters or grills; and protecting drains and other facility intakes and exits. Professional extermination is needed once pests have established. UV light traps can also be used, although they need to be designed to prevent further contamination from the tray that collects the insect remains (Wallin and Haycock, 1998).
2.4 Other Considerations
There is a wide range of issues related to the safety and wholesomeness of food in addition to GMPs. These should be considered in addition to the problems identified at the food processing level when evaluating the effectiveness of food GMPs. They include the following and are discussed in more detail below:
- New trends contributing to foodborne illness,
- Most common causes of foodborne illness,
- High-risk foods, and
- Role of market incentives
New trends contributing to foodborne illness. A number of recent trends contribute to the incidence of foodborne illness. For example, in recent years, there has been an increase in consumer purchases of ready-to-eat (RTE) foods, made popular by the busy lifestyles of people today. Many cases of foodborne illness are caused by RTE foods that were cross contaminated with pathogenic bacteria. Since RTE foods are generally not cooked prior to consumption, the likelihood of foodborne illness is high when these products are contaminated.
Another alerting trend is the increase in new and drug-resistant infectious foodborne agents since the GMPs were last revised. Listeria monocytogenes and Cryptosporidium are examples of newly recognized agents that has been of great concern in the last few years. Some pathogens have also shown antimicrobial resistance, such as Campylobacter jejuni and Salmonella typhimurium DT104. There is also evidence of well-known viruses, such as hepatitis A and Salmonella entertidis, appearing in new foods like produce (Institute of Medicine/National Research Council, 1998). The evolution of these new agents and new vehicles transmitting known pathogens makes prevention of food contamination a moving target for those in charge of ensuring food safety.
The aging population in the United States is another trend of concern: this group is at higher risk for developing illness from contaminated food. As the baby boomer generation enters their retirement years, one can expect this trend to become even more pronounced. These and other changes over time significantly increase the risk of contracting foodborne illness, necessitating a new look at food GMPs in light of these factors.
Most common causes of foodborne illness. Pathogenic bacteria are the most commonly reported agents of foodborne illness, closely followed by viruses (CDC, 2004). Further, most reported cases of foodborne illness are attributable to poor handling at the home or at retail food establishments rather than failures at the food processing level (CDC, 2000). It is not possible to determine (with certainty) the cause of foodborne illness in roughly 50 percent of all foodborne illness cases. Moreover, many foodborne illness cases go unreported.
High-risk foods. The level of risk to public health varies by type of food. Some food products, such as refrigerated RTE foods, have a higher risk of being contaminated by pathogenic bacteria (e.g., Listeria monocytogenes) than others, such as frozen RTE products (NFPA, undated). Further, FDA/CFSAN (2001a) has also shown in their Listeria monocytogenes risk assessment that the level of risk varies for different types of RTE foods. Therefore, from a risk perspective, indiscriminate application and/or recommendation of controls and policies may unduly burden manufacturers as well as the FDA and in some cases lead to inadvertent outcomes. For example, under the current zero-tolerance policy of the Food Safety and Inspection Service (FSIS) for Listeria monocytogenes, when a plant's testing program detects Listeria monocytogenes on plant equipment, the plant is required to recall all product produced on that line during the period of contamination. FSIS may also obtain test data if a plant has a suspected problem with Listeria monocytogenes. While there is a consensus in the industry that aggressive environmental monitoring is essential to controlling Listeria monocytogenes, Tompkin (2002) argues that the zero-tolerance policy discourages, rather than encourages, the RTE food industry from confirming the presence of Listeria monocytogenes in their environmental sampling programs. Many companies may conduct less (rather than more) aggressive environmental monitoring and product testing to avoid regulatory conflict.
Role of market incentives. FSIS is required to inspect meat and poultry slaughtering and processing plants carcass by carcass. As a result of the continuous inspection requirements, FSIS's inspection budget is four times that of FDA (Institute of Medicine/National Research Council, 1998). The lack of inspection resources may contribute to less enforcement of food safety statutes under FDA's jurisdiction. Given the lack of resources, it is important to evaluate the role of other, non-regulatory incentives that encourage food safety. For example, food safety problems can be a major liability for manufacturers of brand name products. If food is said to be unsafe, these manufacturers can face a huge public relations crisis that will negatively affect their bottom line (Ballenger and Ollinger, 2003). Consumers may also shun an entire category of food (Institute of Medicine/National Research Council, 1998). Most producers of branded products, therefore, invest more to ensure the safety of the food they produce. Grocery stores and wholesalers also require strict food safety controls from their suppliers to protect their reputations. For example, USDA's Economic Research Service (ERS) researchers recently surveyed 1,000 slaughtering plants and found that contractual agreements covering food safety standards result in higher levels of food safety with regards to equipment, testing, dehiding, sanitation, and operating procedures (Ballenger and Ollinger, 2003). A similar study for FDA-regulated products may yield comparable results.
References
American Meat Institute (AMI). 2003. Sanitary Equipment Design. AMI Fact Sheet. March.
Ballenger, Nicole, and Michael Ollinger. 2003. Weighing Incentives for Food Safety in Meat and Poultry. Amber Waves. USDA/ERS, Washington, DC. April.
Bissessur, J., K. Permaul, and B. Odhav. 2001. Reduction of Patulin During Apple Juice Clarification. Journal of Food Protection. Vol. 64, No. 8.
Blackburn, Clive de W., and Peter J. McClure. 2002. Foodborne Pathogens: Hazards, Risk Analysis, and Control. CRC Press, Washington, DC.
Boutrif, Ezzeddine. 1999. Minimizing Mycotoxin Risks Using HACCP - The Cracker. International Tree Nut Council. September.
Bryan, Frank L., John J. Guzewich, and Ewen C.D. Todd. 1997. Surveillance of Foodborne Disease III. Summary and Presentation of Data on Vehicles and Contributory Factors; Their Value and Limitation. Journal of Food Protection. Vol. 60, No. 6: 701-714.
CDC. 2004. 2002 Summary Statistics: The Total Number of Foodborne Disease Outbreaks by Etiology. Foodborne Outbreak Response and Surveillance Unit. http://www.cdc.gov/foodborneoutbreaks/us_outb/fbo2002/summary02.htm.
CDC. 2000. Surveillance for Foodborne Disease Outbreaks--United States, 1993--1997. MMWR Surveillance Summaries. Vol. 49 (SS01):1-51. March 17.
Deibel, Kurt, Tom Trautman, Tom DeBoom, William H. Sveum, George Dunaif, Virginia N. Scott, and Dane T. Bernard. 1997. A Comprehensive Approach to Reducing the Risk of Allergens in Food. Journal of Food Protection. Vol. 60, No. 4: 436-441.
FDA/CFSAN. 2002. Food and Drug Administration Pesticide Program: Residue Monitoring 2000. Washington, DC. May.
FDA/CFSAN. 2001a. Draft Assessment of the Relative Risk to Public Health from Foodborne Listeria monocytogenes Among Selected Categories of Ready-to-Eat Foods. January.
FDA/CFSAN. 2001b. Seafood HACCP Alliance HACCP Training Curriculum Manual: Hazards -- Biological, Chemical, and Physical (Chapter 2). November.
FDA/CFSAN. 2001c. Analysis and Evaluation of Preventive Control Measures for the Control and Reduction/Elimination of Microbial Hazards on Fresh and Fresh-cut Produce. September 30.
FDA/CFSAN. 1999a. Potential for Infiltration, Survival, and Growth of Human Pathogens within Fruits and Vegetables. November.
FDA/CFSAN. 1999b. Preliminary Studies on the Potential for Infiltration, Growth, and Survival of Salmonella enterica Hartford and Escherichia coli O157:H7 Within Oranges. November.
FDA/CFSAN. 1999c. Report of 1997 Inspections of Fresh, Unpasteurized Apple Cider Manufacturers. January.
Floyd, Bruce M. 2000. Battling Allergen Contamination. Food Product Design. December.
Folks, Heather, and Dennis Burson. 2001. Food Safety: Chemical Hazards. University of Nebraska Cooperative Extension.
GASGA/CTA. 1997. Mycotoxins in Grains. Technical Leaflet. No. 3. June.
Gregerson, John. 2003. Plain Talk About Allergen Management. Food Processing. January 29.
Gregerson, John. 2002. Third Annual Best Manufacturing Practices Survey. Food Engineering. February.
Gould, William A. 1994. CGMP's/Food Plant Sanitation. CTI Publications, Inc., Timonium, MD.
Higgins, Kevin T. 2003. Food Safety: Say Goodbye to the Burn. Food Engineering. January.
Higgins, Kevin T. 2002. The Culture of Clean. Dairy Foods. November.
Higgins, Kevin T. 2000. A Practical Approach to Allergen Control. Food Engineering. July.
Horne, C.W., L.L. Boleman, C.G. Coffman, J.H. Denton, and D.B. Lawhorn. 1989. Mycotoxins in Feed and Food Producing Crops. U.S. National Dairy Database. http://www.mda.state.mn.us/dairyfood/mfgallergens.htm.
Institute of Medicine. 2001. Food Safety Policy, Science, and Risk Assessment: Strengthening the Connection. Food Forum Workshop Proceedings. National Academy Press, Washington, DC.
Institute of Medicine/National Research Council. 1998. Ensuring Safe Food. National Academy Press, Washington, DC.
Jackson, Lauren S.,, Tina Beacham-Bowden, Susanne E. Keller, Chaitali Adhikari, Kirk T. Taylor, Stewart J. Chirtel, and Robert I. Merker. 2003. Apple Quality, Storage, and Washing Treatments Affect Patulin Levels in Apple Cider. Journal of Food Protection. Vol. 66, No. 4.
Minnesota Department of Agriculture. 2003. Managing Food Allergen Risks. http://www.mda.state.mn.us/dairyfood/mfgallergens.htm.
Morris, Charles E. 2002. Best Practices for Allergen Control. Food Engineering. March.
Moss, Maurice. 2002. Toxigenic Fungi. In Foodborne Pathogens: Hazards, Risk Analysis and Control edited by Clive de W. Blackburn and Peter J. McClure. Woodhead Publishing Limited and CRC Press LLC. Boca Raton, FL.
Moulton, Curtis J. 1992. Reducing Pesticide Residues in Food. Food Safety and Quality. June.
Muth, Mary K., Shawn A. Karns, and Donald W. Anderson. 2001. Analysis of Hazard Analysis Critical Control Point (HACCP) Survey Data. RTI. Prepared for FDA Center for Food Safety and Applied Nutrition. May.
NFPA/AMI/NTF/NCC/NMA/NAMP/SMA/AAMP. 2000. Meat and Poultry Listeria Reassessment Survey. Presentation provided to ERG by Jenny Scott, Senior Director, Food Safety Programs, National Food Processors Association, on May 27, 2003.
National Food Processors Association (NFPA). Undated. Industry Position on Control of Listeria Monocytogenes, With Emphasis on Meat and Poultry Products. National Food Processors Association. www.nfpa-food.org.
Olson, Alan R. 2002. Hard or Sharp Objects. Compendium of Fish, Fishery Product Processes, Hazards, and Controls. October.
Park, Douglas L., Henry Njapau, and E. Boutrif. 1999. Minimizing Risks Posed by Mycotoxins Utilizing the HACCP Concept. Food, Nutrition, and Agriculture. No. 23.
Riordan, D. C. R., G. M. Sapers, T. R. Hankinson, M. Magee, A. M. Mattrazzo, and B. A. Annous. 2001. A Study of U.S. Orchards To Identify Potential Sources of Escherichia coli O157:H7. Journal of Food Protection. Vol. 64, No. 9: 1320-1327.
Stier, Richard F. 2001. Foreign Materials in Foods: Are They Really Dangerous? Market Pulse.
Stopforth, J.D., J. Samelis, J. N. Sofos, P. A. Kendall , and G. C. Smith.. 2002. Biofilm Formation by Acid-Adapted and Nonadapted Listeria monocytogenes in Fresh Beef Decontamination Washings and Its Subsequent Inactivation with Sanitizers. Journal of Food Protection. Vol. 65, No. 11: 1717-1727.
Suttajit, Maitree. 1989. Prevention and Control of Mycotoxins. Mycotoxin Prevention and Control in Foodgrains.
Tilden, John Jr., Wallace Young, Ann-Marie McNamara, Carl Custer, Barbara Boesel, Mary Ann Lambert-Fair, Jesse Majkowski, Dur Vaga, S. B. Werner, Jill Hollingsworth, and J. Glenn Morris. 2002. A New Route of Transmission for Escherichia coli: Infection from Dry Fermented Salami. American Journal of Public Health. Vol. 85, No. 8: 1142-1145.
Tompkin, R.B. 2002. Control of Listeria monocytogenes in the Food Processing Environment. Journal of Food Protection. Vol. 65, No. 4: 709-725.
Tybor, Phillip T., William C. Hurst, A. Estes Reynolds, and George A. Schuler. 1990. Preventing Chemical Foodborne Illness. The University of Georgia College of Agricultural and Environmental Sciences Cooperative Extension Service. November. http://www.ces.uga.edu/pubcd/b1042-w.html.
Wallin, P., and P. Haycock. 1998. Good Manufacturing Practice (GMP). In Foreign Body Prevention, Detection, and Control. Blackie Academic and Professional, London.