Safety and Effectiveness of Gene Therapy

Andrew P. Byrnes, Ph.D.
Office of Tissues and Advanced Therapies
Division of Cellular and Gene Therapies
Gene Transfer and Immunogenicity Branch
Biosketch
Chief, Gene Transfer and Immunogenicity Branch
BS, MS, Yale University, Department of Molecular Biophysics and Biochemistry
PhD, Oxford University, Department of Human Anatomy
Postdoctoral Fellow, Johns Hopkins University, School of Hygiene and Public Health
General Overview
Gene therapy holds great promise for treating cancer, inherited disorders, and other diseases. Gene therapy uses carriers called 'vectors' to deliver genes to tissues where they are needed. Researchers are currently investigating the safety and effectiveness of a variety of different gene therapy vectors in hundreds of clinical trials in the US.
We are studying one type of commonly-used gene therapy vector that is made from a disabled cold virus -- the adenovirus vector. While adenovirus vectors are very efficient at delivering genes, adenovirus vectors are not always easy to target to the correct tissue. In addition, adenovirus vectors can cause toxic effects that limit the amount of vector that doctors can give to patients. We are particularly interested in how to safely deliver large amounts of adenovirus vectors intravenously, with the goal of specifically targeting tumors and other tissues.
New adenovirus gene therapy vectors are tested in animals before human clinical trials begin, and it is important for both researchers and the FDA to know how well these animal studies can predict safety. Thus, another of our major goals is to develop animal models that reliably predict the safety and effectiveness of adenovirus vectors in humans.
Our studies will help us to understand the mechanisms for adenovirus vector targeting and toxicity, and the relevance of animal models to human outcomes. This new knowledge will enable researchers to design safer and more effective gene therapy vectors.
Scientific Overview
Adenovirus (Ad) vectors have shown considerable promise in animal models and are currently being used in numerous clinical trials, especially for the therapy of cancer. We are interested in improving the safety and efficacy of Ad vectors, especially when administered through the vascular system. Certain properties of Ad vectors make them hazardous to administer intravenously in large doses, and our laboratory is trying to understand and fix this problem.
One of our major areas of interest is the innate immune response to Ad vectors. These rapid responses can cause serious toxicity and may severely limit the doses of Ad vectors that are safe to use. In addition, we are also studying how cells in the liver such as Kupffer cells and hepatocytes recognize Ad, since the liver is the major site at which Ad vectors are cleared from the circulation. A better understanding of these mechanisms will help us to develop strategies to improve vector efficacy and reduce toxicity. We will also gain a better understanding of the advantages and disadvantages of using different animal species to predict the behavior of Ad vectors in humans, which is particularly relevant to the regulatory work of the FDA.
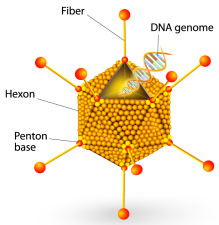
Recent work from our lab and others has shown that Ad vectors are heavily influenced by plasma proteins that rapidly opsonize the vectors after intravenous injection. We found that natural IgM antibodies bind to Ad vectors, activate complement, and reduce liver transduction. Intriguingly, the Ad hexon protein specifically binds to coagulation factor X (FX), and we found that recruitment of FX by Ad vectors protects them against neutralization by complement. These findings show that Ad vectors recruit a number of plasma proteins that interact in complex ways with each other and with cells, and that these host proteins ultimately help to determine whether the vector successfully reaches its target.
In the long run, a better fundamental understanding of Ad vector biology will facilitate the design of safer Ad vectors that are easier to target. Better animal models will be important for testing novel vectors for safety and efficacy.
Publications
- PLoS Pathog 2022 Sep 26;18(9):e1010859
Binding of adenovirus species C hexon to prothrombin and the influence of hexon on vector properties in vitro and in vivo.
Tian J, Xu Z, Moitra R, Palmer DJ, Ng P, Byrnes AP - FEBS Lett 2019 Dec;593(24):3449-60
Interaction of adenovirus with antibodies, complement and coagulation factors.
Allen RJ, Byrnes AP - PLoS One 2018 Feb 5;13(2):e0192353
Hexons from adenovirus serotypes 5 and 48 differentially protect adenovirus vectors from neutralization by mouse and human serum.
Harmon AW, Moitra R, Xu Z, Byrnes AP - Methods Mol Biol 2017;1643:187-96
Evaluating the impact of natural IgM on adenovirus Type 5 gene therapy vectors.
Xu Z, Tian J, Harmon AW, Byrnes AP - J Control Release 2016 Aug 10;235:379-92
Substitution of blood coagulation factor X-binding to Ad5 by position-specific PEGylation: Ppeventing vector clearance and preserving infectivity.
Krutzke L, Prill JM, Engler T, Schmidt CQ, Xu Z, Byrnes AP, Simmet T, Kreppel F - J Virol 2015 Mar;89(6):3412-6
Impact of natural IgM concentration on gene therapy with adenovirus type 5 vectors.
Qiu Q, Xu Z, Tian J, Moitra R, Gunti S, Notkins AL, Byrnes AP