Development of strategies to improve cell therapy product characterization

Steven R. Bauer, Ph.D.
Office of Tissues and Advanced Therapies
Division of Cellular and Gene Therapies
Cellular and Tissue Therapy Branch
Biosketch
Steven R. Bauer, Ph.D., is the Chief of the Cellular and Tissue Therapy Branch (CTTB), Division of Cellular and Gene Therapies (DCGT) in the Office of Tissues and Advanced Therapies (OTAT) at the Center for Biologics Evaluation and Research (CBER), U.S. Food and Drug Administration (FDA). As the chief of CTTB, Dr. Bauer supervises CBER scientific staff engaged in review of cell-based biological therapies, policy development in emerging areas of cellular therapies, and research relevant to their use in clinical trials. He is currently studying mesenchymal stem cell biology and stromal cell-hematopoietic cell interactions that influence development of lymphocytes. Dr. Bauer received his Ph.D. in Biochemistry from the University of Maryland in 1986. From 1986 through 1991, Dr. Bauer was a scientific member of the Basel Institute for Immunology in Basel, Switzerland. In 1991, Dr. Bauer joined CBER’s Division of Cellular and Gene Therapies.
General Overview
Cell-based therapies show great promise for repairing, replacing, restoring, or regenerating damaged cells, tissues and organs. Researchers are working to develop cell-based treatments that are both effective and safe.
Many cell-based therapies use stem cells (SC) that are removed from the body and put into cultures in the laboratory, where they multiply before being infused into the patient. SCs are immature cells that replicate themselves and have the ability to give rise to a variety of different types of cells.
For cell therapies based on embryonic stem cells, stem cells are first stimulated to mature before they are given to a patient. However, embryonic stem cells can cause tumors, so products based on them should not have undifferentiated embryonic stem cells contaminating the product given to patients. Also, more mature cells may be better suited for replacing specific types of damaged or lost cells, or for repairing damaged tissue.
A major challenge posed by SC therapy is the need to ensure their efficacy and safety. Cells manufactured in large quantities outside their natural environment in the human body can become ineffective or dangerous and produce significant adverse effects, such as tumors, severe immune reactions, or growth of unwanted tissue. In response to this challenge, FDA scientists are developing laboratory techniques that will enable the agency to carefully evaluate and characterize these products in order to reliably predict whether they will be safe and effective.
Our laboratories use cell cultures and animal models to develop such techniques and to study the biochemical signals that govern cell behavior during manufacturing and after administration to patients. We are currently using mesenchymal stromal cells, or MSCs, (widely called mesenchymal stem cells) to improve strategies for predicting characteristics of stem-cell based cell products.
These studies will help us develop tests that are practical and applicable to specific manufacturing steps.
Scientific Overview
Our objective is to identify the molecules that exert critical influence on the growth and differentiation of SCs. Such molecules can be used in tests that evaluate and characterize cells during the manufacturing process and as lot-release measurements for cell-therapy products. Lot release tests are done to ensure the safety and quality of products before they are shipped from manufacturing facilities.
Our strategy includes developing tests that reliably quantify the amount of various biological activities that are present in different MSC populations. Using such assays, we found that several biological activities decrease the longer MSCs are cultured, and that MSCs derived from different donors vary in the amount of these activities. The activities that follow this pattern include the ability to generate fat (adipocytes), to mineralize (an indicator of bone formation), and to mediate immunosuppression.
The next step is to identify informative molecular markers--molecules whose presence reflects specific states of activity, disease, response to drugs, potency, and other characteristics of cells and tissues. Once we can quantify specific activities in MSC preparations, we can look for molecular signatures that correlate with the quantities we measure in our biological assays. In order to discover such markers on MSCs, we use a variety of technologies. Our major tools are microarrays (devices that enable the study of the state of activity of tens of thousands of genes at a time), RT-PCR (a technique for rapidly making thousands of copies of pieces of DNA), and flow cytometry (a technique for automatically identifying, counting and examining very large numbers of cells).
Another strategy is to study specific molecules that have been shown to influence development or biological activities of MSCs. We are now studying the role of a protein called DLK in a strain of mouse that is genetically deficient in this protein. In these mice we found that DLK influences the generation of MSCs and their ability to turn into bone and fat. Our studies have also shown that DLK influences development of B-lymphocytes, the immune system cells that produce antibodies.
While using MSCs from this mouse strain to study how DLK helps to control the development of bone and fat tissues, we discovered previously unknown roles that DLK plays in these processes. We've gained important new insights into interactions between MSC cells and the cells that give rise to B lymphocytes.
These studies will likely help us to develop improved methods for testing MSC products to ensure they will be safe and effective when used as therapies. This new knowledge will also help us to discover more informative markers for testing MSC-based products.
Important Links
- Innovation and Regulatory Science- Research Summary: Size and shape of human mesenchymal stem cells may predict future biochemical behavior potentially linked to supporting bone growth
- Innovation and Regulatory Science- Research Summary: Improved Characterization of Bone Marrow Stromal Stem Cells Could Support Their Use as Safe and Effective Therapies
- Innovation and Regulatory Science- Research Summary: Supporting the Development of Mesenchymal Stem Cells as Medical Therapies
- Innovation and Regulatory Science- Scientific Poster: Studies of morphological "signatures" might improve characterization of mesenchymal stem cells for use in tissue regeneration
- Innovation and Regulatory Science- Scientific Poster: Expanded and improved approach to identifying surface protein markers could support development of therapies with human bone marrow multipotent stromal cells
Publications
- Biotechnol Bioeng 2022 Feb;119(2):361-75
Morphological landscapes from high content imaging reveal cytokine priming strategies that enhance mesenchymal stromal cell immunosuppression.
Andrews SH, Klinker MW, Bauer SR, Marklein RA - Am J Physiol Gastrointest Liver Physiol 2021 Apr 1;320(4):G506-20
Stromal DLK1 promotes proliferation and inhibits differentiation of the intestinal epithelium during development.
Ichinose M, Suzuki N, Wang T, Wright JA, Lannagan TR, Vrbanac L, Kobayashi H, Gieniec KA, Ng JQ, Hayakawa Y, García-Gallastegui P, Monsalve EM, Bauer SR, Laborda J, García-Ramírez JJ, Ibarretxe G, Worthley DL, Woods SL - PLoS One 2021 Mar 19;16(3):e0248118
Establishing CD19 B-cell reference control materials for comparable and quantitative cytometric expression analysis.
Wang L, Bhardwaj R, Mostowski H, Patrone PN, Kearsley AJ, Watson J, Lim L, Pichaandi J, Ornatsky O, Majonis D, Bauer SR, Degheidy HA - Cytometry A 2019 Jun;95(6):598-644
Cyt-Geist: current and future challenges in cytometry: reports of the CYTO 2018 conference workshops.
Czechowska K, Lannigan J, Wang L, Arcidiacono J, Ashhurst TM, Barnard RM, Bauer S, Bispo C, Bonilla DL, Brinkman RR, Cabanski M, Chang HD, Chakrabarti L, Chojnowski G, Cotleur B, Degheidy H, Dela Cruz GV, Eck S, Elliott J, Errington R, Filby A, Gagnon D, Gardner R, Green C, Gregory M, Groves CJ, Hall C, Hammes F, Hedrick M, Hoffman R, Icha J, Ivaska J, Jenner DC, Jones D, Kerckhof FM, Kukat C, Lanham D, Leavesley S, Lee M, Lin-Gibson S, Litwin V, Liu Y, Molloy J, Moore JS, Muller S, Nedbal J, Niesner R, Nitta N, Ohlsson-Wilhelm B, Paul NE, Perfetto S, Portat Z, Props R, Radtke S, Rayanki R, Rieger A, Rogers S, Rubbens P, Salomon R, Schiemann M, Sharpe J, Sonder SU, Stewart JJ, Sun Y, Ulrich H, Van Isterdael G, Vitaliti A, van Vreden C, Weber M, Zimmermann J, Vacca G, Wallace P, Tarnok A - Cytotherapy 2019 Jan;21(1):17-31
Morphological profiling using machine learning reveals emergent subpopulations of interferon-gamma-stimulated mesenchymal stromal cells that predict immunosuppression.
Marklein RA, Klinker MW, Drake KA, Polikowsky HG, Lessey-Morillon EC, Bauer SR - Stem Cells Transl Med 2018 Sep;7(9):664-75
Functional profiling of chondrogenically induced multipotent stromal cell aggregates reveals transcriptomic and emergent morphological phenotypes predictive of differentiation capacity.
Lam J, Bellayr IH, Marklein RA, Bauer SR, Puri RK, Sung KE - Cytotherapy 2018 Jun;20(6):779-84
FDA and NIST collaboration on standards development activities supporting innovation and translation of regenerative medicine products.
Arcidiacono JA, Bauer SR, Kaplan DS, Allocca CM, Sarkar S, Lin-Gibson S - Trends Biotechnol 2018 Jan;36(1):105-18
Functionally-relevant morphological profiling: a tool to assess cellular heterogeneity.
Marklein RA, Lam J, Guvendiren M, Sung KE, Bauer SR - SLAS Technol 2017 Dec;22(6):646-61
Adaptation of a simple microfluidic platform for high-dimensional quantitative morphological analysis of human mesenchymal stromal cells on polystyrene-based substrates.
Lam J, Marklein RA, Jimenez-Torres JA, Beebe DJ, Bauer SR, Sung KE - Proc Natl Acad Sci U S A 2017 Mar 28;114(13):E2598-607
Morphological features of IFN-gamma-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity.
Klinker MW, Marklein RA, Lo Surdo JL, Wei CH, Bauer SR - Nat Genet 2016 Dec;48(12):1473-80
Fetus-derived DLK1 is required for maternal metabolic adaptations to pregnancy and is associated with fetal growth restriction.
Cleaton MA, Dent CL, Howard M, Corish JA, Gutteridge I, Sovio U, Gaccioli F, Takahashi N, Bauer SR, Charnock-Jones DS, Powell TL, Smith GC, Ferguson-Smith AC, Charalambous M - Oncotarget 2016 Sep 20;7(38):60986-99
Role of mir-15a/16-1 in early B cell development in a mouse model of chronic lymphocytic leukemia.
Underbayev C, Kasar S, Ruezinsky W, Degheidy H, Schneider JS, Marti G, Bauer SR, Fraidenraich D, Lightfoote MM, Parashar V, Raveche E, Batish M - Stem Cells Dev 2016 Jun 1;25(11):861-73
Identification of predictive gene markers for multipotent stromal cell proliferation.
Bellayr IH, Marklein RA, Lo Surdo JL, Bauer SR, Puri RK - Stem Cells 2016 Apr;34(4):935-47
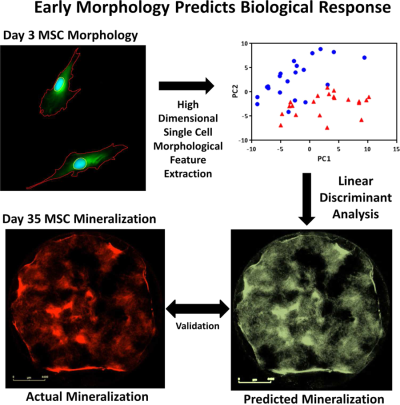
High content imaging of early morphological signatures predicts long term mineralization capacity of human mesenchymal stem cells upon osteogenic induction.
Marklein RA, Lo Surdo JL, Bellayr IH, Godil SA, Puri RK, Bauer SR - PLoS One 2016 Mar 9;11(3):e0149331
Alterations in the mir-15a/16-1 loci impairs its processing and augments B-1 expansion in de novo mouse model of chronic lymphocytic leukemia (CLL).
Kasar S, Underbayev C, Hassan M, Ilev I, Degheidy H, Bauer S, Marti G, Lutz C, Raveche E, Batish M - Cytometry B Clin Cytom 2016 Mar;90(2):159-67
Consistent, multi-instrument single tube quantification of CD20 in antibody bound per cell based on CD4 reference.
Degheidy H, Abbasi F, Mostowski H, Gaigalas AK, Marti G, Bauer S, Wang L - Cytotherapy 2016 Mar;18(3):336-43
Chromosomal stability of mesenchymal stromal cells during in vitro culture.
Stultz BG, McGinnis K, Thompson EE, Lo Surdo JL, Bauer SR, Hursh DA - Curr Protoc Cytom 2016 Jan 6;75:1.29.1-1.29.14
Quantitative flow cytometry measurements in antibodies bound per cell based on a CD4 reference.
Wang L, Degheidy H, Abbasi F, Mostowski H, Marti G, Bauer S, Hoffman RA, Gaigalas AK - Data Brief 2015 Nov 1;5:864-70
The proteomic dataset for bone marrow derived human mesenchymal stromal cells: effect of in vitro passaging.
Mindaye ST, Lo Surdo J, Bauer SR, Alterman MA - Stem Cell Res 2015 Oct 30;15(3):655-64
System-wide survey of proteomic responses of human bone marrow stromal cells (hBMSCs) to in vitro cultivation.
Mindaye ST, Surdo JL, Bauer SR, Alterman MA - Stem Cells 2015 Jul;33(7):2169-81
Chromatin changes at the PPAR-γ2 promoter during bone marrow-derived multipotent stromal cell culture correlate with loss of gene activation potential.
Lynch PJ, Thompson EE, McGinnis K, Rovira Gonzalez YI, Lo Surdo J, Bauer SR, Hursh DA