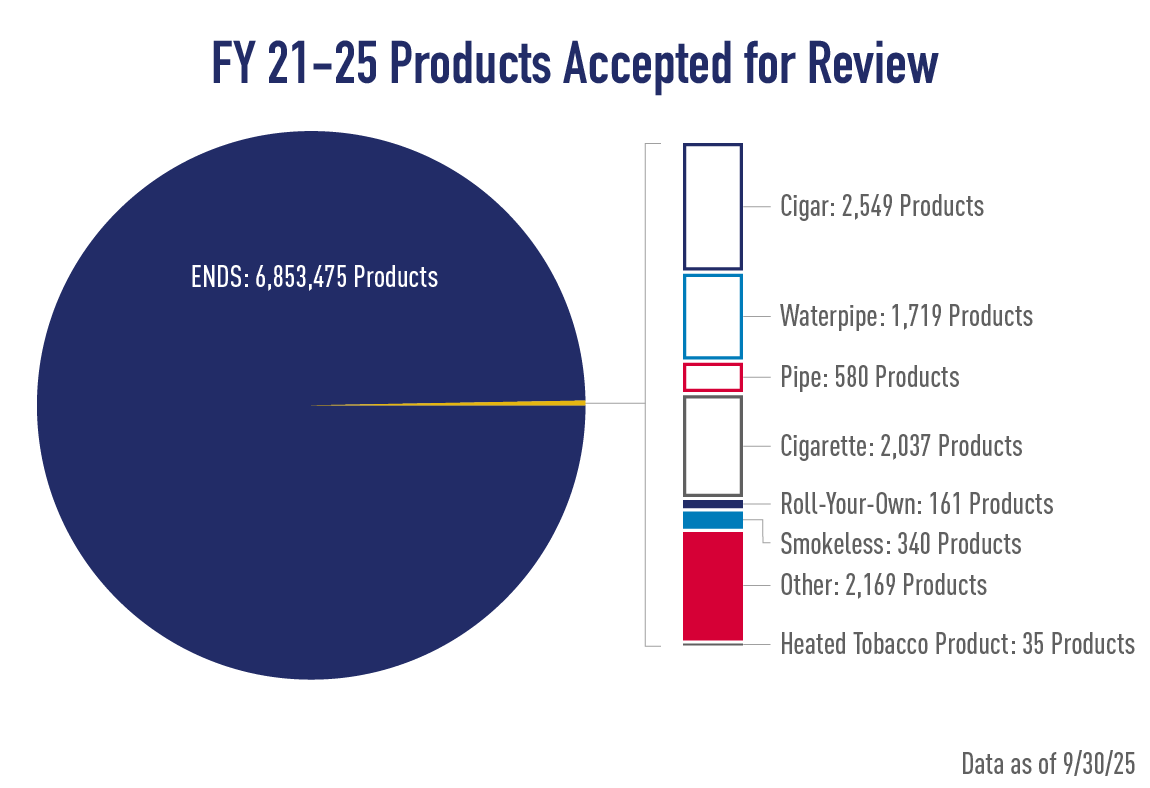
Tobacco Product Applications: Metrics & Reporting
On this page:
- Premarket Tobacco Product Applications (PMTA)
- Substantial Equivalence (SE) Reports
- Exemption from Substantial Equivalence Requests (EX REQ)
- Tobacco Product Applications Metrics Glossary
FDA regularly updates this page with reporting and progress of FDA intermediate and final actions taken on premarket applications – PMTA, SE Report and EX REQ – across the application review process.
FDA intends to provide these metrics and data on a regular and reliable basis and in an easy-to-understand format, typically within a month of the closing of the reporting period.
Note: Information on this page is current as of Septemer 30, 2025.
FDA may periodically reassess and change the categories or amount of data provided on this website. This data is produced on an ongoing basis and is subject to change due to updates, corrections, or other reasons. Some metrics can also change as FDA is processing an extremely large number of applications that move through many steps during the review process. The data reported here is generally accurate to within 10%.
Most of the metrics reported reflect the number of tobacco products that are at each stage. Descriptions of each of the metrics are provided within the Tobacco Product Applications Metrics Glossary.
FDA has received some submissions that could not be processed and ingested for various reasons, including but not limited to lack of required forms, use of an incorrect form, or use of an incorrect format on the application. Given that FDA was unable to fully process these submissions, they were issued Refuse to Accept Letters or withdrawn by the applicant. Since complete information was not available, metrics for these submissions are not provided in the reported tables.
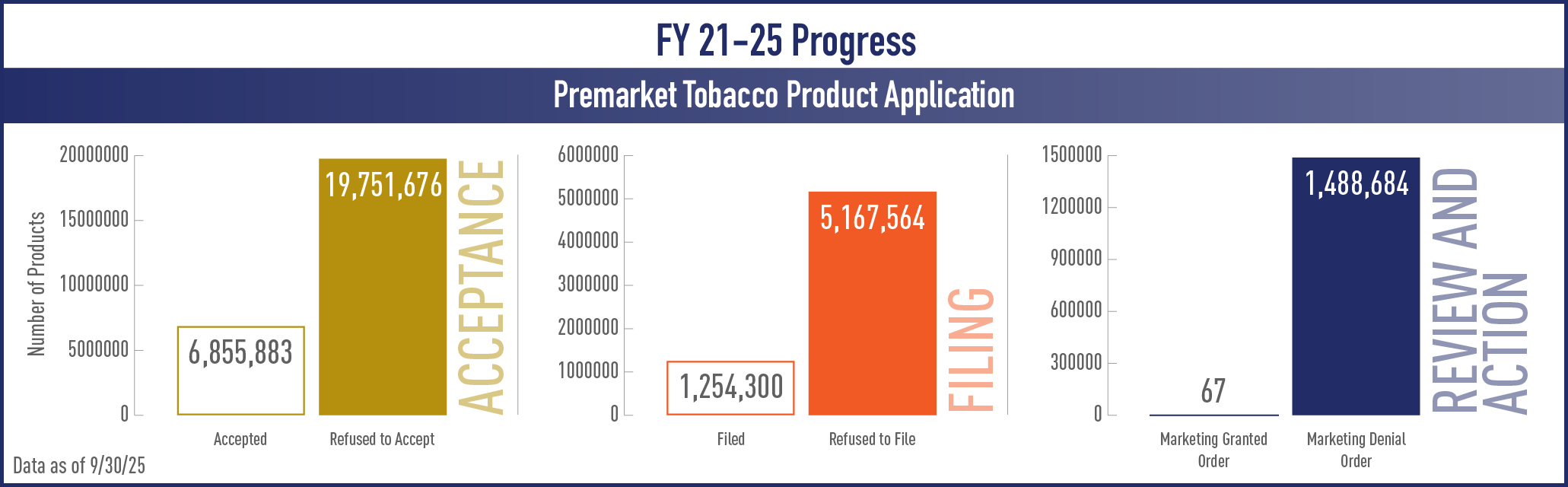
Premarket Tobacco Product Applications (PMTA)
View the complete set of PMTA Metrics* for Acceptance, Filing and Review/Action Phases
Additional Resources
- Actions on Tobacco Product Marketing Applications From CY 2013 to FY 2020
- Searchable Tobacco Products Database
| Metric | Definition |
|---|---|
| Accepted | Number of products for which CTP performed an administrative review, accepted the application for further review, and issued an Acceptance letter to the applicant |
| Refuse to Accept | Number of products for which CTP did not accept a marketing application because a preliminary administrative review concludes that it has not met a minimum threshold for acceptability for FDA review |
| Total Applications Received | Number of products for which an application was received through one of the three submission types [SE Report, EX REQ or PMTA] |
| Filed | Number of products for which CTP performed a preliminary scientific review of a PMTA, did not identify significant data or information missing, and issued a Filing letter to the applicant informing them that they will be entering substantive review |
| Refuse to File | Number of products for which CTP will not file a marketing application because a preliminary scientific review concludes that necessary information is not included in the application |
| Number of Discipline Reviews Completed | Number of discipline reviews conducted by CTP for evaluation of the scientific information and data in a marketing application. Review disciplines may include engineering, chemistry, microbiology, toxicology, social science, behavioral and clinical pharmacology, medical, epidemiology, regulatory, and compliance |
| Deficiency Letters Issued | Number of products for which CTP issued deficiency letters requesting additional information from the applicant to make a marketing decision |
| Environmental Information Request Letters Issued | Number of products for which CTP made a scientific decision to support a marketing order; however, applicant still is required to provide information for environmental considerations before a marketing order can be issued |
| Marketing Granted Order | Number of products for which CTP concluded that marketing a new tobacco product is appropriate for the protection of public health and CTP issued an order indicating that the new product can be marketed |
| Marketing Denial Order | Number of products for which CTP concluded that marketing a new tobacco product is not appropriate for the protection of public health and CTP issued an order denying marketing of the new product |
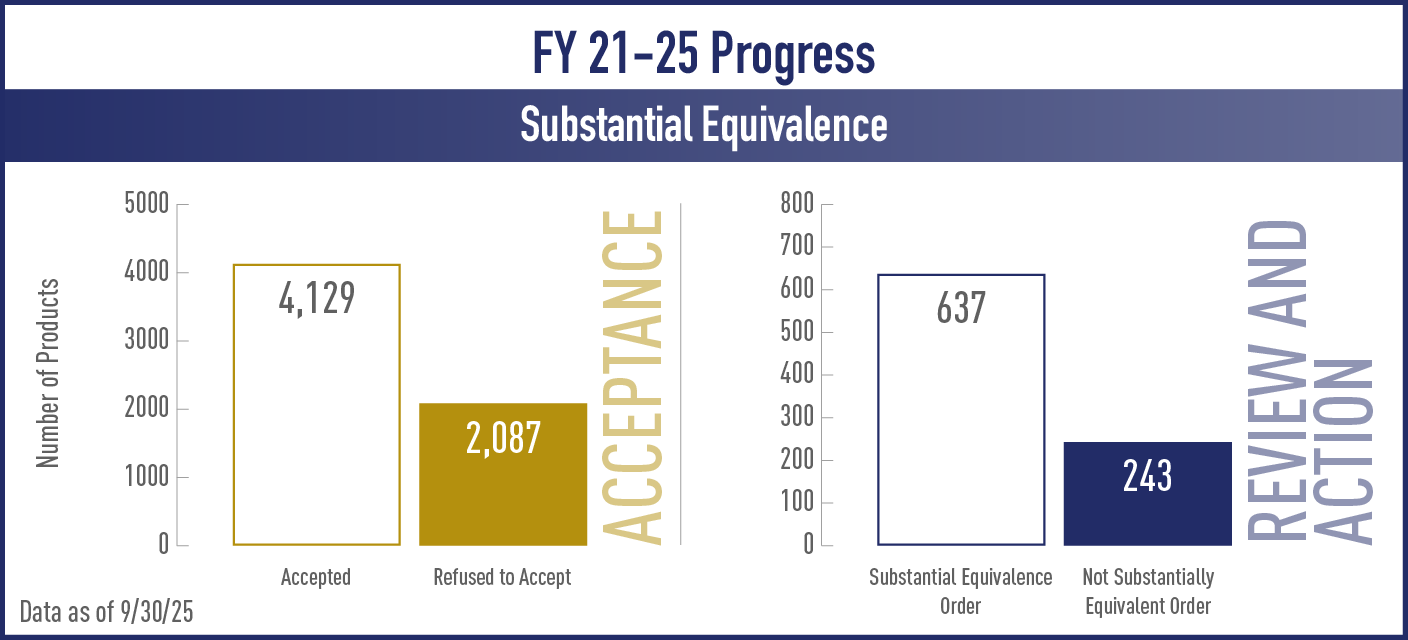
| Substantial Equivalence Order | Number of products for which CTP concluded that a new product is substantially equivalent to a predicate product and CTP issued an order indicating that the new product can be marketed |
| Not Substantially Equivalent Order | Number of products for which CTP concluded that a new product is not substantially equivalent to a predicate product and CTP issued an order denying the marketing of a new product |
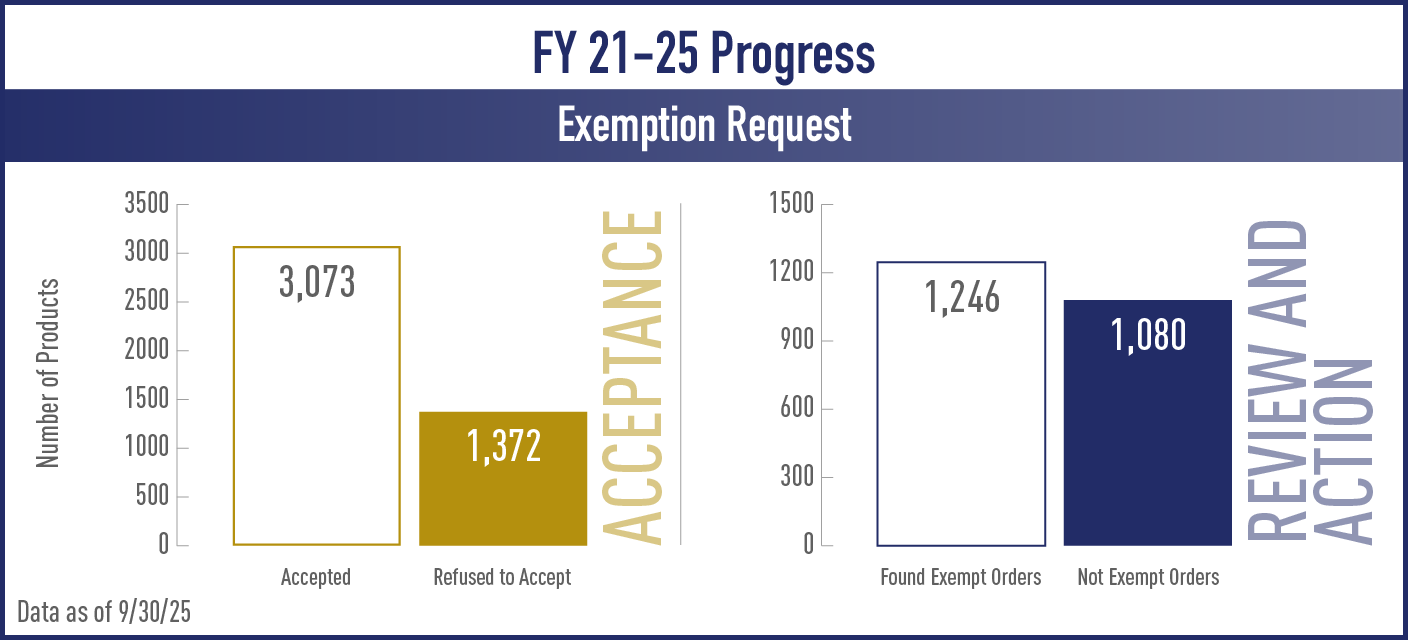
| Found Exempt Order | Number of products for which CTP concluded that a new product is exempt from the requirements of substantial equivalence and CTP issued an order indicating that the new product can be marketed |
| Not Exempt Order | Number of products for which CTP concluded that a new product is not exempt from demonstrating substantial equivalence to the original product and CTP issued an order denying marketing of the new product |
| Withdrawn by Applicant | Number of products for which CTP acknowledged and complied with the applicant’s request to remove its marketing application from consideration for a marketing order |
| Closure | Number of products for which CTP did not make a decision on an application because there was an error and the marketing application should not have been opened |