2023 FDA Science Forum
The role of FDA’s Veterinary Laboratory Investigation and Response Network in monitoring antimicrobial resistance of animal pathogens
- Authors:
- Center:
-
Contributing OfficeCenter for Veterinary Medicine
Abstract
Background:
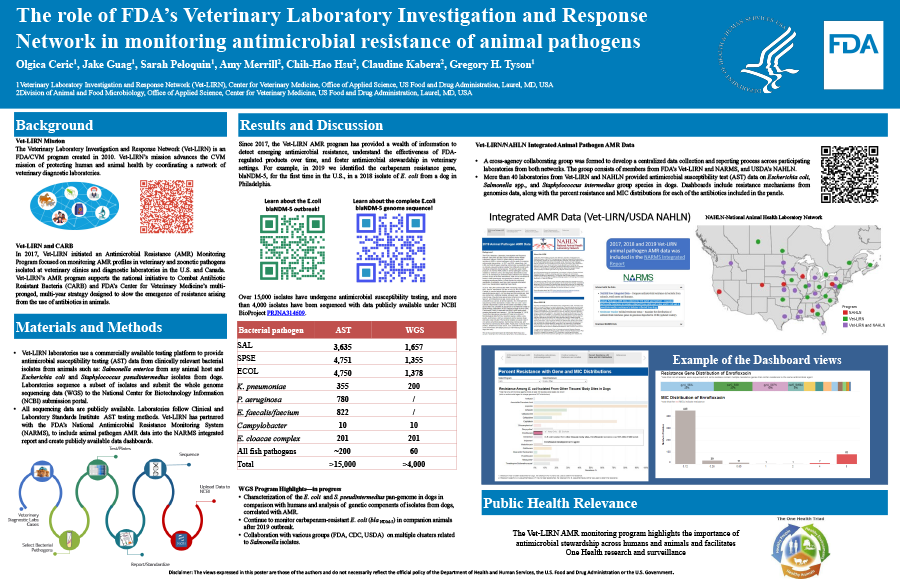
The Veterinary Laboratory Investigation and Response Network (Vet-LIRN) is an FDA program created in 2010 to coordinate a network of veterinary diagnostic laboratories. In 2017, Vet-LIRN initiated an Antimicrobial Resistance (AMR) Monitoring Program focused on monitoring AMR profiles in veterinary and zoonotic pathogens isolated at veterinary clinics and diagnostic laboratories in U.S. and Canada. Vet-LIRN’s AMR program supports the national initiative to Combat Antibiotic Resistant Bacteria (CARB) and FDA’s Center for Veterinary Medicine multi-pronged, multi-year strategy designed to slow the emergence of resistance arising from the use of antibiotics in animals.
Methods:
Vet-LIRN laboratories provided antimicrobial susceptibility testing (AST) data from clinically relevant bacterial isolates from animals: Salmonella enterica from any animal host, Escherichia coli and Staphylococcus pseudintermedius isolates from dogs, using a commercially available testing platform. Laboratories sequenced a subset of isolates and submitted the whole genome sequencing data (WGS) to the National Center for Biotechnology Information (NCBI) submission portal, where they were assembled and analyzed using NCBI’s bioinformatics pipelines. All sequencing data are publicly available. Laboratories follow Clinical and Laboratory Standards Institute AST testing methods. Vet-LIRN has partnered with the FDA’s National Antimicrobial Resistance Monitoring System (NARMS), to include animal pathogen AMR data into the NARMS integrated report and create publicly available data dashboards.
Results:
Since 2017, the Vet-LIRN AMR program has provided a wealth of information to detect emerging antimicrobial resistance, understand the effectiveness of FDA-regulated products over time, and foster antimicrobial stewardship in veterinary settings. For example, in 2019 we identified the carbapenem resistance gene, blaNDM-5, for the first time in the U. S., in a 2018 isolate of E. coli from a dog in Philadelphia. Over 15,000 isolates have undergone antimicrobial susceptibility testing, and more than 4,000 isolates have been sequenced with data publicly available under NCBI BioProject PRJNA314609. Vet-LIRN AMR data dashboards include resistance mechanisms from genomics data, along with the percent resistance and MIC distributions for each of the antibiotics included in the panels.
Conclusions:
By making AMR monitoring data publicly accessible, the Vet-LIRN AMR monitoring program facilitates international One Health research and surveillance.
Download the Poster (PDF; 0.83 MB)
