2023 FDA Science Forum
One-year physical stability study of in-house sunscreen formulations
- Authors:
- Center:
-
Contributing OfficeCenter for Drug Evaluation and Research
Abstract
Background
Sunscreen creams are usually formulated as oil in water emulsions. It is a system where an oil phase is evenly dispersed as globules in a continuous aqueous phase. The active ingredients in sunscreen are UV filters, which protect the skin by absorbing UV radiations at the skin surface. It has been shown that chemical UV filters penetrate the skin and result in systemic exposure. There are several factors that may affect the skin absorption including formulation characteristics and excipient compositions.
Purpose
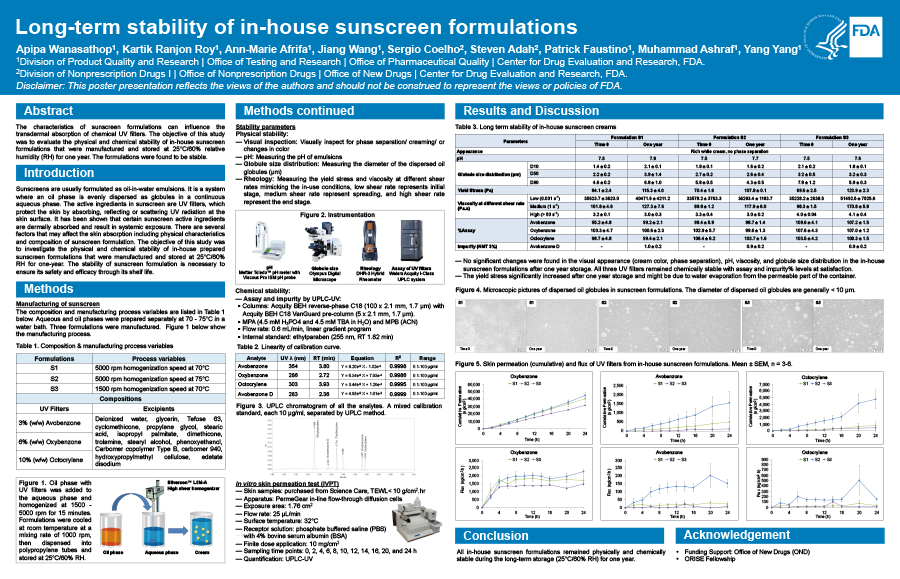
The objective of this study was to investigate the one-year physical stability of in-house prepared sunscreen formulations that were manufactured and stored at 25°/60% RH. Understanding the formulation stability will ensure that the formulation is safe and effective throughout its shelf life. Methodology: A series of in-house sunscreen formulations were prepared by systematically varying the manufacturing process parameters. These process parameters may influence formulation characteristics and lead to different levels of dermal absorption of UV filters. The creams (oil in water emulsion) containing 3% (w/w) avobenzone, 10% (w/w) octocrylene, and 6% (w/w) oxybenzone as chemical UV filters were prepared and stored at 25°C/60% RH. The physical stability was determined by measuring the globule size distribution, viscosity, and pH. An in-vitro skin permeation test (IVPT) will be performed with stable formulations to evaluate the dermal absorption of the chemical UV filters.
Results
Preliminary results showed that an increase in homogenizer speed decreased the globule size distribution, and all the manufacturing process parameters (temperature, speed, and time) appeared to have an impact on the viscosity of formulations. However, after storage for one year, no significant changes were found in the visual appearance (cream color, phase separation), pH, viscosity, and globule size distribution in the sunscreen formulations. Conclusion: In-house prepared sunscreen formulations were physically stable during one-year storage period. Therefore, the formulations can be further tested for chemical stability (e.g., assay) and skin absorption.