2021 FDA Science Forum
FDA Postmarketing Requirements in Pregnant and Lactating Women: Past, Present, and Future
- Authors:
- Center:
-
Contributing OfficeCenter for Drug Evaluation and Research
Abstract
Background
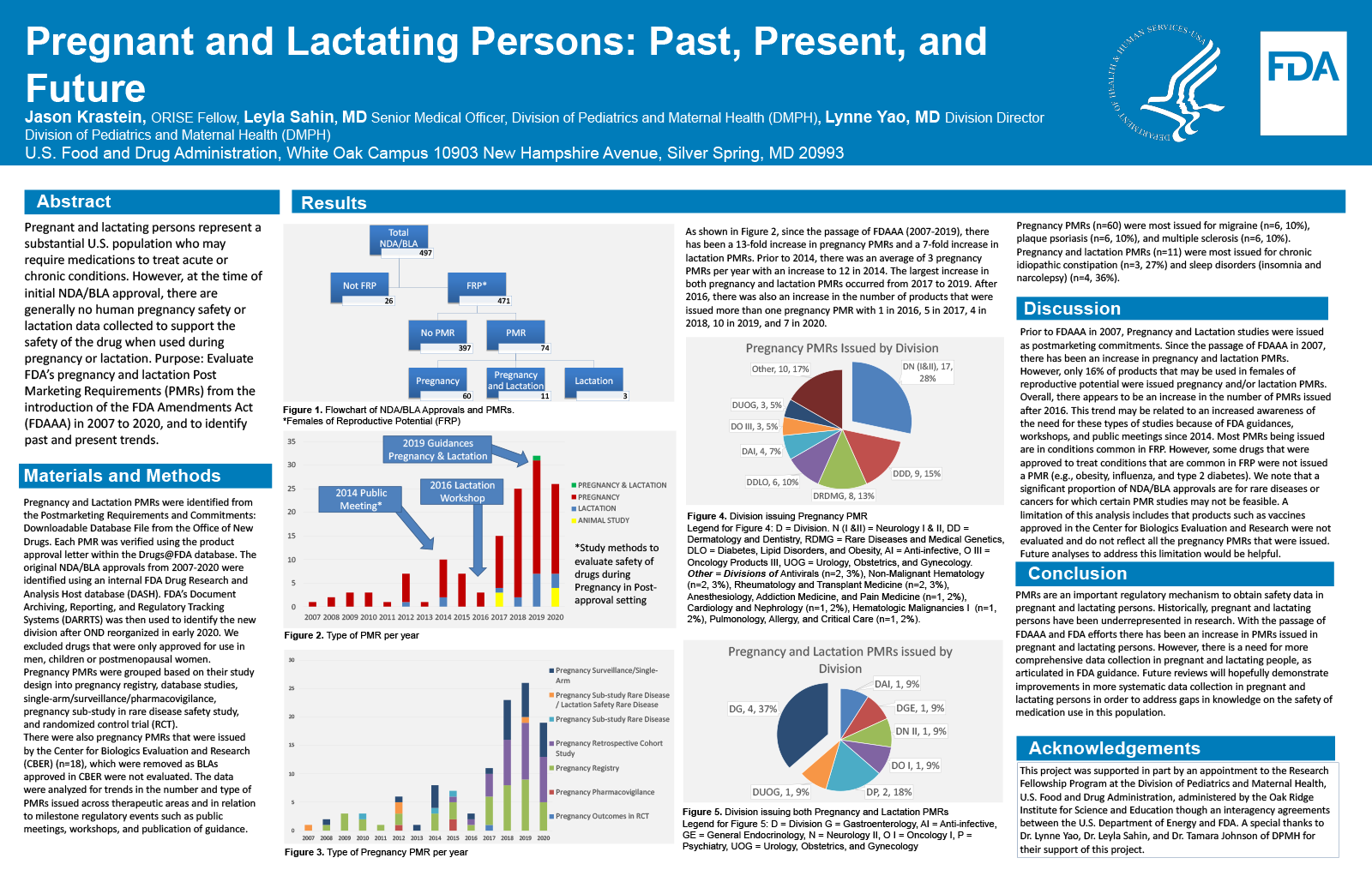
Pregnant and lactating women represent an important segment of the population and may have chronic or acute conditions. However, at the time of initial NDA/BLA approval, there are generally no human pregnancy safety or lactation data collected to support the safety of the drug when used during pregnancy or lactation.
Purpose
Evaluate FDA’s pregnancy and lactation Post Marketing Requirements (PMRs) from the introduction of the FDA Amendments Act (FDAAA) in 2007 to 2020, and to identify past and present trends.
Methods
Pregnancy and Lactation PMRs were identified from the Postmarketing Requirements and Commitments: Downloadable Database File from the Office of New Drugs. Each PMR was verified using the product approval letter within the Drugs@FDA database. The original NDA/BLA approvals from 2007-2020 were identified using an internal FDA database. The data were analyzed for trends in the number and type of PMRs issued across therapeutic areas and in relation to milestone regulatory events such as public meetings and publication of guidance.
Results
We identified 488 original NDA/BLA approvals from 2007-2020. Of the total number of approvals, 95% (462) were drugs that may be used in females of reproductive potential (FRP). Of those that may be used in FRP, 17% (85) were issued a pregnancy or lactation PMR. The most common drugs to receive a pregnancy PMR (n=71) were for migraine (n=6, 9%), plaque psoriasis (n=7, 10%), and multiple sclerosis (n=6, 9%). The most common drugs to receive a lactation PMR (n=14) were for sleep disorders (insomnia and narcolepsy) (n=4, 29%), and chronic idiopathic constipation (n=4, 29%). Since the passage of FDAAA, there has been a 6.5-fold increase in pregnancy PMRs and a 7-fold increase in lactation PMRs.
Conclusion
Because safety data in pregnant and lactating women are generally collected post approval, PMRs are an important regulatory mechanism to obtain safety data in pregnant and lactating women. Since 2007, there has been an increase in pregnancy and lactation PMRs. However, PMRs in these populations are disproportionally lower than in the general population. These is a need for more comprehensive data collection in pregnant and lactating women, as articulated in FDA guidance.