Testimony | In Person
Event Title
Implementation of the Generic Drug User Fee Amendments of 2012 (GDUFA) - House Testimony
April 1, 2016
Testimony of
Janet Woodcock, M.D.
Director, Center for Drug Evaluation and Research
U.S. Food and Drug Administration
Before the
House Committee on Oversight and Government Reform
February 4, 2016
Introduction
Chairman Chaffetz, Ranking Member Cummings, and Members of the Committee, I am Dr. Janet Woodcock, Director of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA or the Agency), which is part of the Department of Health and Human Services (HHS). Thank you for the opportunity to be here today to discuss FDA’s implementation of the Generic Drug User Fee Amendments of 2012 (GDUFA).
Historically, the generic drug program has been a great success.
The generic drug industry has grown from modest beginnings into a major force in health care. According to the IMS Institute for Healthcare Informatics, generic drugs now account for 88% of prescriptions dispensed in the United States, and saved the U.S. health system $1.68 trillion from 2005 to 2014.
This success brought new challenges.
Over the last several decades, the generic industry, the number of generic drug applications (known as “Abbreviated New Drug Applications” or “ANDAs”) submitted to FDA for review, and the number of foreign facilities making generic drugs grew substantially. As a result, FDA’s generic drug program became increasingly under-resourced. Its staffing did not keep pace with the growth of the industry.
Because the program could not keep up with its workload, a backlog of submitted ANDAs developed and grew. It overwhelmed the FDA staff and created unpredictability and delay for industry.
Solution: GDUFA
After multiple attempts, FDA and the generic industry developed a proposal for a generic drug user fee program and submitted it to Congress. Congress enacted it as part of the Food and Drug Administration Safety and Innovation Act of 2012.
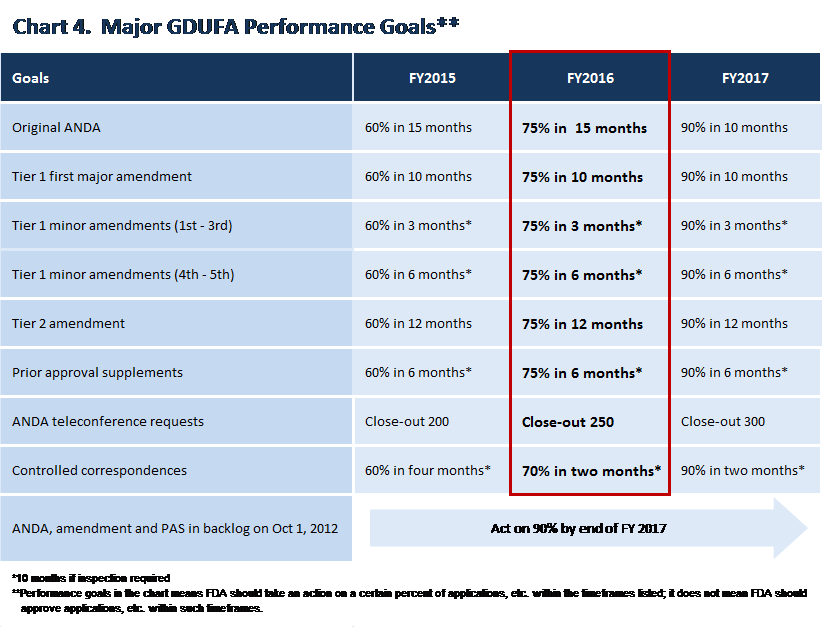
Under GDUFA, industry agreed to pay approximately $300 million in fees each year of the 5 year program. In exchange, FDA committed to performance goals, the specifics of which are contained in the Generic Drug User Fee Act Program Performance Goals and Procedures agreement that was negotiated with industry (“GDUFA Commitment Letter”)1. Because of the amount of hiring, restructuring, and catch-up needed, performance goals were set to commence in the later years of the program. The GDUFA performance goals with respect to ANDAs, amendments to ANDAs, and prior approval supplements (PAS)2 are timeframes by which FDA is to take a “first action” on an application, by either granting an approval or tentative approval3, or, if there are deficiencies that prevent approval, identifying those deficiencies to the applicant in a complete response letter or in a refusal to receive4 the application. When deficiencies are identified, industry usually responds by correcting them and resubmitting the application.
To date, FDA has met or exceeded all performance goals outlined in the GDUFA Commitment Letter.
Actions on Pre-GDUFA (“Backlog”) Applications
A major commitment of GDUFA was to take a “first action” on 90% of the “backlog” applications, defined as pre-GDUFA applications pending before the Agency on October 1, 2012, by the end of Fiscal Year 2017. As of October 1, 2012, the backlog included 2866 ANDAs and 1873 PASs. As Chart 5 indicates, to date, FDA has completed first actions on 84% of ANDAs and 88% of PASs. And so, FDA is well ahead of schedule in achieving the GDUFA goal to significantly reduce the backlog, and our ultimate goal of eliminating it.
Some of these backlog applications had been pending or in review for a long time prior to GDUFA. At this point in time, as FDA acts on one of the outstanding backlog applications, the “time to approval” of such application will be recorded as, at minimum, 40 months (i.e., we now are three years and four months (40 months) into GDUFA implementation). This helps to explain the often-quoted 42 month approval time, which does not apply to post-GDUFA applications as explained below.
Moreover, the filing backlog for ANDAs has been eliminated. “Filing” is where we evaluate if a drug sponsor’s submitted application is sufficiently complete to permit FDA’s substantive review. In August 2014, we had a filing backlog of over 1,100 applications. Now that backlog is gone.
Actions on Post-GDUFA Original Applications5
In addition to the pre-GDUFA backlog applications, nearly 2,500 applications were submitted in FY 2013 and FY 2014 after GDUFA had commenced. Per the GDUFA Commitment Letter, these FY 2013 and FY 2014 applications have no GDUFA goal dates. Notwithstanding this, FDA assigned internal goals, called “Target Action Dates” (TADs), to both the pre-GDUFA backlog applications and to the FY 2013 and FY 2014 applications and has been aggressively reviewing them.
Under the GDUFA Commitment Letter, applications submitted in FY 2015 have a 15 month “first-action” goal date. Goal dates represent a paradigm shift. They substantially improve the speed and predictability of review. So, any concerns about delayed competition in the generic space pertain to prior years, when our backlog was accumulating, and not to applications with GDUFA goal dates.
Importantly, if the ANDA submission is a potential “first generic” or could mitigate a drug shortage, its review is expedited. The performance goals for those generic applications submitted in the first few months of FY 2015 are just coming due. We are on track to meet or exceed our obligations under the GDUFA Commitment Letter relative to these applications and already have approved or otherwise acted on some applications submitted in FY 2015.
Applications submitted in Fiscal Year 2016 also have a first-action goal date of 15 months, with the Agency committed to reviewing a greater percentage of generic applications within the timeframe specified.
The cumulative result of all this effort is a huge increase in the productivity of the generics program. As Chart 7 indicates, we ended last year at a new monthly high of 99 approvals and tentative approvals in December.
Of course, a major goal of GDUFA is timely approval of affordable, high-quality generic drugs. FDA’s success in implementing the Prescription Drug User Fee Amendments (PDUFA) program—the user fee program for new drugs begun in 1992—provided the Agency with valuable experience that enabled us to rapidly build a modern generic drug review process once sufficient resources were made available through user fees. FDA is now on track to achieve the throughput needed, with sustained levels of record or near-record approvals in the third and fourth quarter of 2015.
Prioritization of First Generics Applications
We recognize that certain types of applications merit priority attention based on their public health significance.
For example, we consider “first generics” to be public health priorities, as they can lead to increased patient access. First generics are just what they sound like—the first generic versions of a drug to enter the market. Under GDUFA, beginning in FY 2015, each of these first generic submissions automatically receives a 15 month goal date. FDA has worked hard to provide an even faster review for potential first generics. Because they are public health priorities, we expedite their review, like an express lane at the supermarket.
Thanks to GDUFA, we made substantial first generic program improvements. We opened a docket to solicit technical input; issued a public-facing, transparent prioritization policy;6 formed a team to expedite the review of first generics; trained review staff; and enhanced our computer systems to streamline the process.
Potential first generics are approximately 15% of our overall workload. All of these have been going in the “express lane.” Over the past 3 years we have approved hundreds of first generics for over 200 new drug products. Significant first generic approvals for 2015, and the indications (abbreviated) for which these products were approved, are listed on the next page.
Significant First Generic Approvals for 2015
Brand (Generic name)
Abilify® (aripiprazole)
Fusilev® (levoleucovorin)
Enablex® (darifenacin)
Lotronex® (alosetron)
Zyvox® (linezolid)
Tygacil® (tigecycline)
Vagifem® (estradiol)
Integrelin® (eptifibatide)
Xenazine® (tetrabenazine)
Indications (Abbreviated)
Schizophrenia, Bipolar Disorder
Supports cancer treatment
Overactive bladder
Irritable bowel syndrome
Pneumonia, serious infections
Pneumonia, serious infections
Menopause
Heart attack
Huntington’s Disease
Progress on Additional Important GDUFA Goals
In addition to reducing the backlog, acting on post-GDUFA applications, and approving first generics, FDA is also achieving other important GDUFA goals.
One goal addressed risk-based inspection parity for foreign and domestic facilities. Before 2012, the law required us to inspect domestic facilities at a two-year interval, but was silent on frequency for foreign establishments, regardless of their relative risk. GDUFA directs us to target inspections globally on the basis of risk. We are on track to achieve the goal of risk-based inspection parity between foreign and domestic facilities by the end of FY 2017.
GDUFA also established goals for our review of PASs. PASs are important because they enable flexibility and improvements for generic drug manufacturing. To date, we have substantially exceeded GDUFA PAS goal of 60% reviewed within 6 months if an inspection is not required and 10 months if an inspection is required.
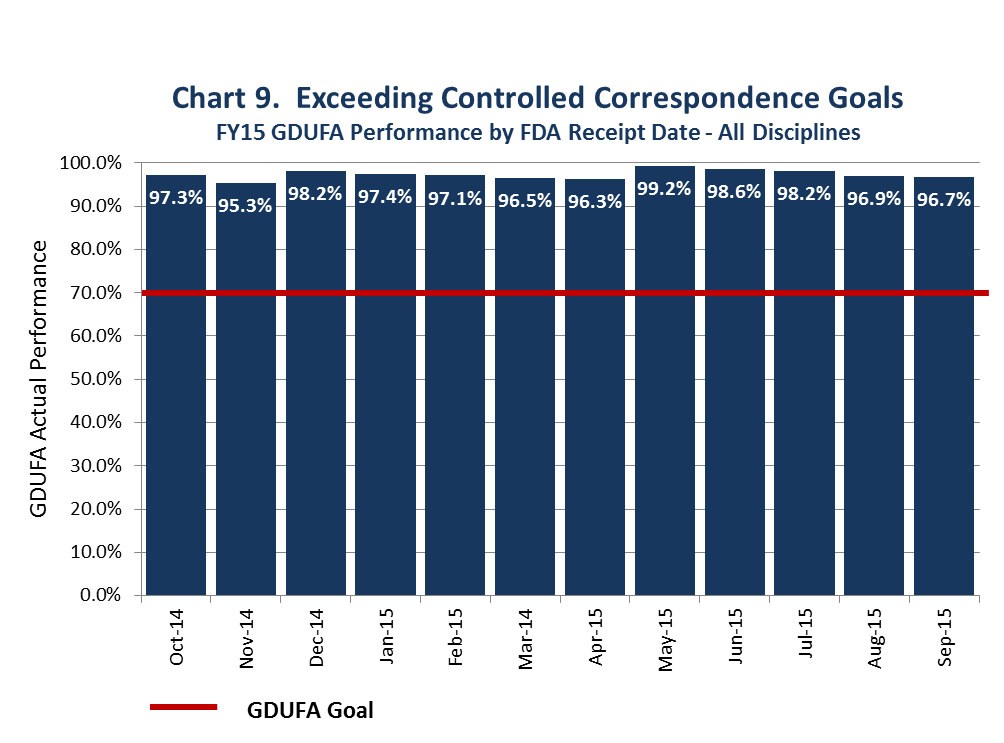
There are also GDUFA goals for responding to controlled correspondence. Controlled correspondences are product development questions that FDA answers to help companies develop applications. The GDUFA goal for FY 2015 was to respond to 70% within 4 months of submission. As noted in Chart 9, we substantially exceeded our commitments in this area.
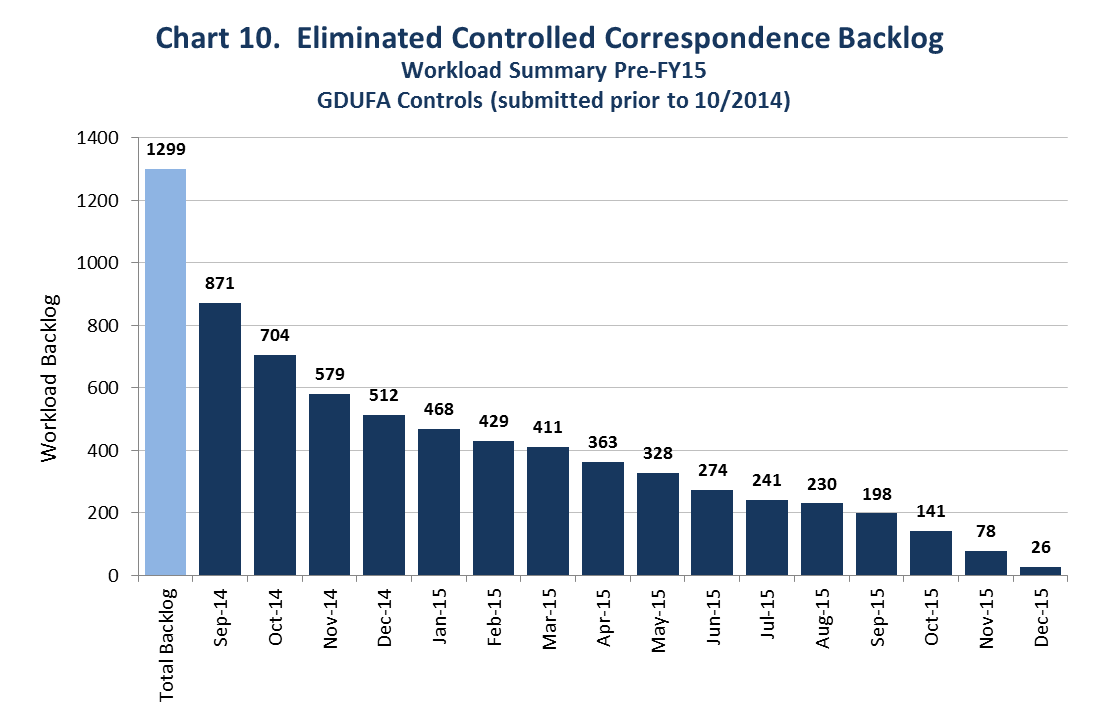
We also had a significant backlog of controlled correspondence from before goal dates started. We have eliminated that backlog.
How did FDA achieve these results?
Deep, foundational restructuring.
We achieved these results by building a modern generic drug program.
This involved major reorganizations. We reorganized the Office of Generic Drugs and elevated it to “Super-Office” status, on par with the Office of New Drugs. We established a new Office of Pharmaceutical Quality7 to integrate the quality components of the review.
We developed an integrated informatics platform to support the generic drug review process. It is a significant improvement over our fragmented, legacy systems, and has enhanced our productivity.
We hired and trained over 1,000 new employees, achieving our GDUFA hiring goals well ahead of schedule.
Flexible Approach: Communications and Transparency
We also took a flexible approach to managing the program in ways that benefit generic drug sponsors and, ultimately, patients.
One example of fine-tuning the process to speed approvals is the “Information Request” process. As originally agreed during the GDUFA negotiations, FDA was to package all deficiencies found in the review of an application and provide them to the applicant in a complete response letter. But that turned out not to be a helpful approach and industry asked us to send them information concerning individual deficiencies on a rolling basis, instead of consolidating them all into one package. This would help industry correct deficiencies in “real time.” We agreed. In FY 2015, we issued over 4,700 Information Requests.
At industry’s request, we communicated “Target Action Dates” (TADs). As previously described, TADs are our internal deadlines for action on all applications without goal dates. Although GDUFA did not require the Agency to develop TADs or communicate them to industry, we understand that they help companies plan product launches, spurring timely access to generics.
We also reacted to much larger than expected ANDA submission volume. As the GDUFA Commitment Letter stated, GDUFA review goals and planning were based on the assumption that the Agency would receive approximately 750 ANDAs per year. We budgeted and planned with this projection in mind. However, in FYs 2012, 2013 and 2014, we received over 1,000, nearly 1,000, and nearly 1,500 applications, respectively. We had to modify our planning and execution accordingly.
In addition, we increased our output of product-specific guidances. These guidances clarify our expectations concerning specific products so industry can develop and obtain approval of generic versions of branded drugs more quickly.
Ongoing Challenges
We do have some ongoing challenges. The first relates to submission quality. Historically, it has taken on average about 4 review cycles to approve an ANDA as a result of deficiencies by generic drug sponsors in submitting complete and quality applications (see Chart 15). This has resulted in the submission of numerous amendments to correct deficiencies in the original ANDAs and comprises a huge amount of re-work for FDA and industry alike. Currently, for example, nearly 900 applications are back with industry awaiting resubmission to correct deficiencies in the original applications. New filing policies will help, but more work by both the Agency and industry will be necessary to have the filings be “right the first time.”
As noted in the public minutes8 published as part of the GDUFA II negotiations now underway, FDA and industry are discussing a pre-ANDA process by which FDA and industry would address approval challenges for particular drugs prior to ANDA submissions, which could make a big difference in the completeness and quality of applications.
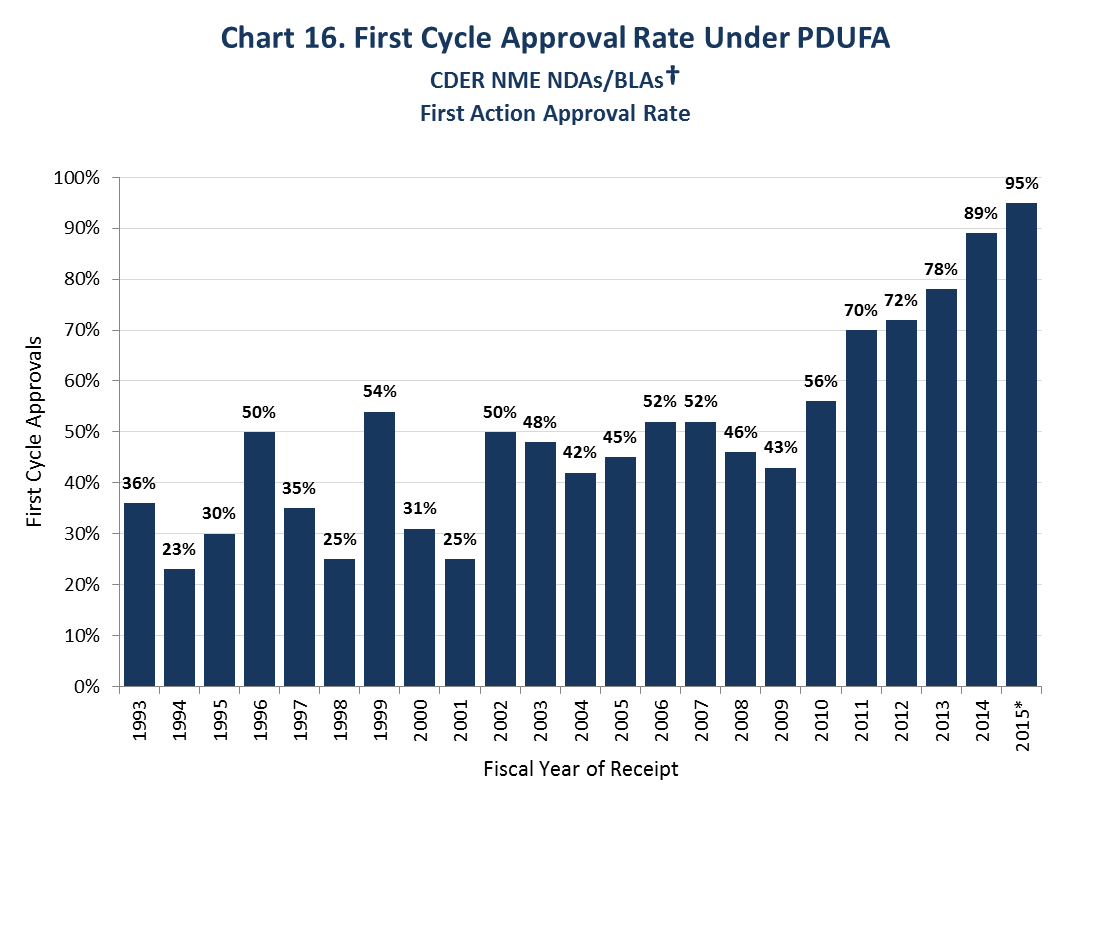
Improvement may take some time. As Chart 16 shows, in the first few years of the PDUFA program, the first cycle approval rate dropped as low as 23%. Now it is 95%. Achieving this was the result of many years of work on standards and expectations.
Second, there is a need for more research in the generics space. Some drugs lack generic competition because there is no convincing bioequivalence test method available. In these instances, a more extensive clinical study is needed to show equivalence of a generic to a brand name drug. Similarly, methods for showing chemical sameness for certain complex drugs are not available. GDUFA provided funding for research efforts to work out these problems. So far, GDUFA has funded $34.9 million in research programs that will open up previously blocked pathways. However, scientific research takes time, and results will need to be translated into guidance for industry.
Third, shared system Risk Evaluation and Mitigation Strategies—or REMS—pose challenges. REMS are used to ensure that the benefits of drugs outweigh their risks. The statutory requirement that REMS programs that include elements to assure safe use (ETASU) be implemented through a “single shared system” relies on brand and generic companies to agree on such a system before generic drugs may come to market. This is challenging to implement and frequently results in blocking generic competition. We would welcome the opportunity to discuss possible solutions to this problem with you.
Fourth, to better assure quality in an increasingly globalized industry, FDA is undertaking major changes in quality regulation. CDER’s Office of Pharmaceutical Quality, FDA’s Program Alignment Group9 and the International Council for Harmonisation10 are all driving major changes, and FDA is pursuing mutual reliance discussions with the European Union. As a result of this work and collaborative effort, the public can be assured that FDA will hold generic products to the same quality standards as brand drugs, no matter where they are manufactured or tested.
Conclusion
I am extremely proud of what the FDA staff has accomplished in implementing GDUFA. Getting to where we are today has taken an enormous amount of work and above-and-beyond dedication by many people over the past three years. I have no doubt that we will exceed the goals initially established for this program.
GDUFA II discussions between the Agency and Industry are underway and constructive. We are excited and positive about the opportunity to make significant program improvements.
Thank you for the opportunity to describe what we’ve accomplished over the past three years. I look forward to your questions.
- Generic Drug User Fee Act Program Performance Goals and Procedures
- A prior approval supplement is a post approval change requiring supplemental submission and approval prior to distribution of the product made using the change.
- Tentative approval applies if a generic drug product is otherwise ready for approval before the expiration of any patents or exclusivities accorded to the reference listed drug product. In such instances, FDA issues a tentative approval letter to the applicant. FDA delays final approval of the generic drug product until all patent or exclusivity issues have been resolved. A tentative approval does not allow the applicant to market the generic drug product.
- A “refuse-to-receive” decision indicates that FDA determined that an ANDA is not sufficiently complete to permit a substantive review.
- In this context, “Original Applications” refer to the first ANDA submitted, as opposed to a subsequent amendment or supplement to the ANDA.”
- FDA/CDER Manual of Policies and Procedures: Office of Generic Drugs
- Office of Pharmaceutical Quality
- GDUFA Reauthorizatoin Negotiation Sessions
- FDA Program Alignment
- International Council for Harmonisation website
--])