FDA’s Generic Drug Program in 2020 Helped Ensure Availability of High-Quality, Affordable Drugs Amid COVID-19

By: Sally Choe, Ph.D., Director, Office of Generic Drugs, Center for Drugs Evaluation and Research
Generic drugs play a vital role in facilitating access to lifesaving medicines for Americans and remain a public health priority for the U.S. Food and Drug Administration. Right now, there are more than 10,000 FDA-approved generic drugs and nine out of 10 prescriptions in the U.S. are filled by generics. Generic drugs have saved the health care system close to $2.2 trillion dollars in the past decade. That is good news for the health of Americans.
Amid the challenges of a worldwide pandemic and rapidly advancing science, the FDA’s generic drug program continued steadfast efforts to help increase the availability of safe, effective, high-quality, more affordable drugs in the U.S. Altogether in 2020, the FDA’s generic drug program moved steadily forward even as the COVID-19 pandemic presented additional complexities, resulting in new realities and unique challenges.
Generic Drugs During a Pandemic
Last year, as the COVID-19 public health emergency unfolded, the agency quickly pivoted to making generic drug submissions involving potential treatments and supportive therapies for patients with COVID-19 the top priority. Generic medicines, such as intravenous drugs for patients placed on ventilators and steroids that helped reduce COVID-19 deaths, were used to help fight the effects of the virus on patients.
The FDA’s Office of Generic Drugs (OGD) identified and implemented tools and strategies to confront the pandemic, including the creation of a system for identifying generic drugs critical to the treatment of patients with COVID-19, and then subsequently taking regulatory and scientific action to accelerate their assessment. This system for identifying generic drugs to address the COVID-19 public health emergency involved establishing infrastructure and resolving assessment issues to support prompt actions involving critically-needed generic drugs.
The FDA also worked diligently to support manufacturers of already approved generic drug products who needed to make changes to manufacturing processes or facilities to address disruptions caused by the pandemic. The generic drug products they produced included antibiotics, sedatives for ventilated patients, anticoagulants, and pulmonary medications, among others. We developed regulatory and scientific approaches to help efficiently restart generic drug development programs interrupted by the pandemic. For instance, we directly assisted applicants with bioequivalence studies impacted by the global pandemic, including answering their questions about protocol revisions and information collection.
FDA’s OGD Continued Work to Improve Access to More Affordable Medicines
While simultaneously working on the response to COVID-19, the FDA made significant progress last year with daily efforts to improve access and reduce the cost of medicines by helping to support competition. Carrying on our regular, non-COVID related work, we were encouraged to see how our past efforts to improve the generic drug program’s efficiency, quality, and predictability added to our resilience with supporting rapid approval of safe, effective generic medicines for use in fighting the sudden occurrence of the pandemic.
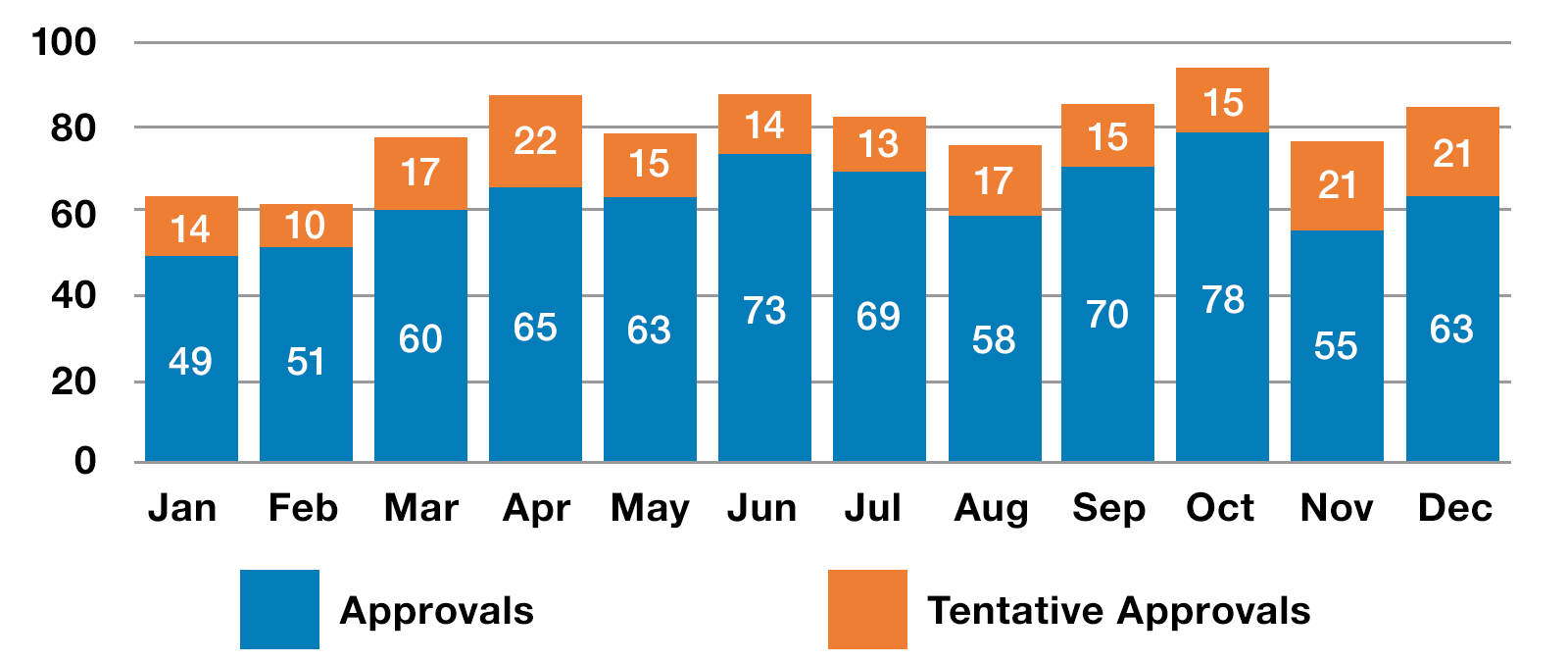
In 2020, we approved or tentatively approved 948 generic drug applications, called Abbreviated New Drug Applications (ANDAs), including 72 first generics. We also approved 30 generics under the Competitive Generic Therapy (CGT) pathway, including a quarterly record of 17 CGT approvals in the first quarter of fiscal year 2020.
Generic drug approvals can have a real impact for American patients. For example, for the first time, patients with multiple sclerosis now have a generic of Tecfidera (dimethyl fumarate) capsules, patients with toxoplasmosis now have a generic of Daraprim (pyrimethamine) tablets, and patients experiencing severe hypoglycemia now have a generic of glucagon for injection.
OGD’s proactive and collaborative approaches to supporting generic drug development and assessment can lead to the development and submission of higher quality generic drug applications and therefore more timely access to safe, effective generics. One important tool used to communicate with prospective generic drug applicants is called controlled correspondence. A controlled correspondence inquiry is submitted to the agency by (or on behalf of) a generic drug manufacturer or related industry, requesting information on a specific element of generic drug product development. In 2020, OGD responded to 3,711 controlled correspondence inquiries submitted by industry – a record!
Another tool is the Pre-ANDA Program, which was established under the Generic Drug User Fee Amendments of 2017 (GDUFA II). This program provides product development assistance to generic drug developers through written communications and meetings that help clarify regulatory expectations early in the development process and during application assessment. This program provides a special focus on complex generic drug products, such as some inhaled or injectable products, which can be more challenging for generic drug developers to copy, often leading to a lack of generic competition even after patents and exclusivities no longer block generic drug approval. In 2020, we received 121 product development and pre-submission pre-ANDA meeting requests.
Beyond directly approving generic drugs, OGD continued its implementation of the FDA’s Drug Competition Action Plan (DCAP) by taking steps to: improve the efficiency of the generic drug development, assessment, and approval process; maximize scientific and regulatory clarity with respect to complex generic drugs; and close loopholes that allow brand name drug companies to “game” FDA rules in ways that delay the generic competition Congress intended. This included publication of guidances for industry, Manual of Policy and Procedures, and other policy actions.
2020 Generic Drugs Approved and Tentatively* Approved
For example, public dockets were opened and guidances were issued to seek user feedback on how to enhance the Orange Book, which celebrated its 40th anniversary in 2020. We look forward to making this key resource as useful as possible for stakeholders, including any improvements that can be made to advance the agency’s goal of improving access to safe, effective, high-quality, and affordable treatment options for Americans. Our related efforts are listed on the DCAP web page, which is updated regularly.
We also continued to leverage OGD science and research through recommendations in general and in product-specific guidances (PSGs). PSGs can help applicants develop generic drugs that can meet our high approval standards. Recommendations in PSGs help make industry’s research and development decisions more efficient and cost-effective by identifying the most appropriate methodology and evidence needed to support a specific generic drug’s comparison with the brand name drug.
In another effort to support the development of complex generics, in August 2020, the FDA announced an award of a five-year grant from the GDUFA Science and Research Program that went to the Universities of Maryland and Michigan to establish the Center for Research on Complex Generics. The $5 million grant aims to enhance research collaborations with the generic drug industry to further OGD’s mission of increasing access to safe, effective, high-quality, and more affordable generic drug products. This goal will be pursued through collaborative research, training, workshops, laboratory projects to meet development needs, a Complex Generics Scholars program, and exchange of resources between the FDA, the generic drug industry, and stakeholders.
OGD’s work also extended beyond our borders to ensure Americans continue to have access to lower cost, safe, effective, and high-quality medicines. Last year, we maximized our involvement in the International Pharmaceutical Regulators Programme by sharing information with more than 30 regulatory agencies and through harmonization initiatives to promote scientific and regulatory exchange and identify topics for potential international harmonization efforts for generic drugs. We also continued to leverage and strengthen bilateral partnerships with Health Canada and the European Medicines Agency to advance collaborative efforts intended to address complex scientific and regulatory issues for generic drugs.
The FDA’s 2020 Office of Generic Drugs Annual Report provides a comprehensive look at what was accomplished and illustrates how the agency is well-positioned to continue its critical work in 2021.
Because of the commitment of the OGD staff along with everyone in the Center for Drug Evaluation and Research, and across the FDA, the generic drug program is stronger than ever, even during a time of unprecedented global health challenges. We remain steadfast in our dedication to seamlessly taking timely actions to assess and approve generic drugs on behalf of American patients and consumers.