TAP Overview
FDA TAP Advisors: Proactive and Strategic Engagement
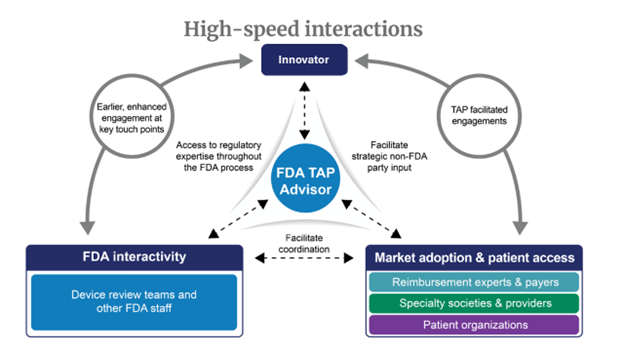
TAP advisors facilitate engagement with FDA and non-FDA parties that can help program participants identify strategic options to streamline the path to patient access to their devices. With TAP, the FDA is striving to play a more active part in supporting patient access innovative, safe and effective devices.
In their roles at the FDA, TAP advisors:
- Offer expertise across many fields, including regulatory information and device development challenges and solutions.
- Help identify key challenges that may hinder the path to U.S. marketing authorization through proactive and holistic discussions.
- Facilitate discussions with non-FDA parties that can help TAP innovators best strategize on actionable solutions for potential challenges related to market adoption, clinical use, and patient access.
FDA Interactions: A New Paradigm of Speed and Collaboration
Through TAP, CDRH is applying lessons learned from the COVID-19 public health emergency to move quickly and take a more fluid and informal approach to interacting with the MedTech industry. TAP is also designed to offer increased predictability and transparency in the regulatory process for participating companies.
Multiple accelerated FDA engagement options are available to TAP innovators, including:
- Regular informal touch-base meetings with TAP advisor and review teams.
- Teleconferences on requested topics within 14 days.
- Written feedback on requested topics within 40 days (accelerated to 21 days for biocompatibility or sterility topics).
Patient Access: Pursuing Our Public Health Mission
TAP strives to accelerate patient access to high-quality, safe, effective, and innovative medical devices. TAP can help innovators prepare for the road ahead and consider the full path to market and patient access for their enrolled devices. TAP advisors also act as innovation ambassadors and can help identify non-FDA entities from the MedTech ecosystem who may provide input to TAP innovators. Early insights and non-FDA party input gained through TAP can help increase the likelihood that a high-quality, safe, and effective device ultimately reaches patients.
Non-FDA Parties include:
- patient organizations
- medical specialty societies and associations
- reimbursement experts and payers
TAP Limitations
TAP focuses on identifying risks, challenges, and potential solutions during device development, while facilitating interactions between TAP innovators and non-FDA parties to support more widespread patient access to high-quality, safe, effective, and innovative devices.
TAP does not:
- Determine what kind of risks are acceptable.
- Decide what activities should or should not be financed.
- Formulate or execute strategies.
- Replace professional management or consultants.
Ultimately, TAP innovators must decide on their own strategies, evidence needs, and execution plans.