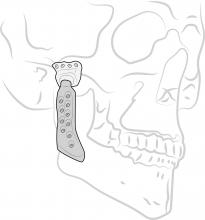
Temporomandibular Joint (TMJ) Implants
Temporomandibular joint (TMJ) implants are intended to be surgically implanted in the jaw to replace the temporomandibular joint. A variety of artificial materials have been used to partially or totally replace the TMJ, including plastics, Teflon, silicone, metals, and a combination of these materials.
On this page:
- About TJM Implants
- Candidates for TMJ Implants
- Problems Reported with TMJ Implants
- Recommendations for Patients
- How the FDA Engages with TMJ Patients
- Additional Resources
About TMJ Implants
The TMJ implant may take many different forms. Some implants are intended to replace only a part of the joint, such as the temporomandibular joint disc or the glenoid fossa, and other implants are intended to replace the entire joint.
The purpose of these types of implants is to restore the TMJ function, including opening and closing the mouth and chewing. However, all movements of a normal joint may not be possible with an artificial joint.
Candidates for TMJ Implants
TMJ implants are intended for patients who do not respond to other forms of treatment, including non-surgical treatment, or for whom prior joint reconstruction, including tissue grafts and/or joint replacement, failed to restore function and relieve symptoms. The National Institute of Dental and Craniofacial Research (NIDCR) says, "less is often best in treating TMJ disorders." Discuss all treatment options with your healthcare provider.
Candidates for TMJ implants include:
- Patients who have undergone non-surgical treatments and at least one previous surgical procedure.
- Patients whose previous surgeries may have resulted in failed joint reconstruction. This includes patients who have undergone bone graft procedures using bone grafts derived from their patient's own bone tissue or from synthetic bone. Often these patients may also have experienced numerous surgeries to one or both of their temporomandibular joints.
- Patients who have had inflammatory or immunological responses, including end-stage TMJ deterioration, which may preclude further reconstruction with bone from the patient.
- Patients with severe pain and extremely limited function, including patients with severe trauma to the temporomandibular joint, neoplasms (tumors), congenital deformities, ankylosis, or arthritis involving the TMJ, rendering the joint dysfunctional.
Problems Reported with TMJ Implants
As with all medical devices, the FDA receives medical device reports (MDRs) related to problems or concerns with TMJ implants. Approximately 5,500 TMJ total joint replacement procedures were performed in U.S. from 2005 to 2014 (Journal of Oral and Maxillofacial Surgery, Volume 74, Issue 8, 1531 – 1538). The FDA received 680 MDRs related to TMJ implants from 2014-2018, prompting increased engagement with patients living with this condition, evaluation of patient-reported outcome measures, and review of postmarket studies.
The most frequent patient problems reported in MDRs include pain, swelling, infection, headache, limited movement of the implanted joint, excessive bone growth, increased sensitivity, disability, and the need for additional surgical procedures (including revision) and non-surgical treatments or therapy.
Report a Problem with a TMD Device
Recommendations for Patients
The FDA provides these recommendations for patients considering TMJ implants and for patients who have already received implants.
Find a health care provider who is knowledgeable about temporomandibular disorder (TMD) symptoms and treatment options. When choosing a health care provider, you may wish to consider their training, experience, board certification, their patient follow-up, and your own comfort level.
To find qualified professionals near you, you can contact:
- Academic or research centers, such as a dental school at a university,
- Your state dental association
- The American Academy of Oral Medicine
- The American Association of Oral and Maxillofacial Surgeons (AAOMS)
Notify the FDA if you are having problems with your TMJ implant. If you are having problems with your TMJ implant, refer to Reporting TMD Device Problems to the FDA.
Make sure that you can receive notifications about any problems with your TMJ implants. Notifications may come from the manufacturer to you or to your health care provider. Accordingly, be sure to contact the doctor who performed your TMJ implant surgery or the manufacturer of your implant if you have a change in contact information (such as address or phone number).
You may also proactively monitor the manufacturer’s website and social media as well as www.FDA.gov. The best way to stay informed about medical device recalls is to subscribe to the Medical Device Safety and Recalls email list.
If you receive a safety notification for your TMJ implants or notice of a recall (removal or correction) from the manufacturer or the FDA, please contact your physician to discuss how this may impact your medical care.
How the FDA Engages with TMD Patients and Patient Organizations
The FDA engages with patients to better understand how TMD and the medical devices used to diagnose, manage, monitor or treat TMD impacts their lives.
Forming Partnerships with Patient Organizations
The FDA participated in the first Patient-Led TMJ Roundtable Meeting in June 2016. Patients with TMD shared their perspectives about current TMD treatments, expressed the need for more interdisciplinary research, and suggested a paradigm shift in TMJ disorders treatment.
As a result of this roundtable meeting, four working groups were formed to enable patients, health care providers, researchers, and regulators to collaboratively discuss ways to advance treatments for this condition. This roundtable led to an ongoing partnership with the FDA that has benefitted the TMD community and the FDA.
A second Patient-Led Roundtable Meeting was held in May 2018. During this meeting, the four working groups reported on their progress with identified gaps and data needs in addressing TMD diagnosis and treatment and established an approach for making progress. For details, see The TMJ Patient-Led Round Table: A History and Summary of Work.
Involving Patients in Measuring TMJ Device Outcomes
The roundtable efforts highlighted the importance of understanding the tools available to measure the patient experience living with TMD so that these tools could be used in the evaluation of medical devices.
The FDA is partnering with TMD patients to potentially develop a research tool that evaluates the symptoms (such as pain) and functions (such as speaking or chewing) associated with TMD. The Patient-Reported Outcome (PRO) tool will enable researchers to measure what is important to patients in the evaluation of medical devices.
Additional Resources
- National Institute of Dental and Craniofacial Research. Health Info: TMJ (Temporomandibular Joint and Muscle Disorders)
- American Association of Oral and Maxillofacial Surgeons (2017). Statement by the American Association of Oral and Maxillofacial Surgeons Concerning the Management of Selected Clinical Conditions and Associated Clinical Procedures: Temporomandibular Disorders
- American Dental Association. TMJ (video)
- The TMJ Association a nonprofit, patient advocacy organization whose mission is to improve the quality of health care and lives of everyone affected by temporomandibular disorders
- Gauer & Semidey (2015). Diagnosis and Treatment of Temporomandibular Disorders. Am Fam Physician, 91, 378-386.
- American Academy of Orofacial Pain (AAOP)
- International Association for the Study of Pain (IASP)