TactiFlex Ablation Catheter, Sensor Enabled – P220013
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: TactiFlex Ablation Catheter, Sensor Enabled
PMA Applicant: Abbott Medical
Address: 5050 Nathan Lane North, Plymouth, MN 55442
Approval Date: May 18, 2023
Approval Letter: Approval Order
What is it?
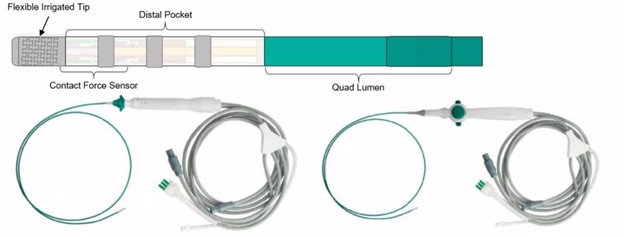
The TactiFlex Ablation Catheter is a long, flexible wire with a metal electrode at its tip. The tip can be heated and is intended to be used as a treatment for atrial fibrillation, an abnormal heart rhythm causing fast and irregular heartbeats, and type I atrial flutter, another abnormal heart rhythm that also causes fast heartbeats (tachycardia).
How does it work?
A doctor inserts the catheter through a vein in the groin (upper leg). The doctor positions the catheter tip at the opening of a vein that carries blood from the lungs to the heart, called a pulmonary vein. The doctor then turns on the catheter tip, heating up, or ablating, the heart tissue in order to block abnormal electrical signals from the pulmonary vein to the heart. Those abnormal electrical signals are what causes the abnormal heart rhythm. The doctor moves the catheter to the next pulmonary vein. The process is repeated until all pulmonary veins are treated. The catheter can also be used to heat or ablate other areas of the heart’s upper chambers that may be causing the abnormal heart rhythms.
When is it used?
The TactiFlex Ablation Catheter is approved for use in people who have atrial fibrillation episodes that last less than seven days (paroxysmal atrial fibrillation) and whose episodes are not corrected with medicine.
What will it accomplish?
In a clinical study, doctors used the TactiFlex Ablation Catheter, Sensor Enabled to treat 334 people with paroxysmal atrial fibrillation and 113 people with typical (type I) atrial flutter. The patients were followed for 12 months including a 3-month period for medicine adjustments and re-treatment. At the end of 12 months, atrial fibrillation, atrial flutter, and atrial tachycardia were not detected in 247 of the participants.
When should it not be used?
The device should not be used in people who:
- Had heart surgery where an incision was made into at least one of the heart’s chambers within the last 4 weeks.
- Have tumors or clots inside their heart.
- Have artificial heart valves.
- Have baffles or patches between the left and right upper chambers (atria) of the heart.
- Have active bloodstream, or systemic, infections.
- Cannot receive heparin or an alternative to prevent clotting (anticoagulation).
Additional information (including warnings, precautions, and adverse events):
- Summary of Safety and Effectiveness Data (SSED)
- Labeling
- PMA Database entry