Senza Spinal Cord Stimulation System – P130022/S039
This is a brief overview of information related to the FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data and product labeling for more information on this product, its indications for use, and the basis for the FDA’s approval.
Product Name: Senza Spinal Cord Stimulation (SCS) System
PMA Applicant: Nevro Corporation
Address: 1800 Bridge Parkway, Redwood City, CA 94065
Approval Date: July 16, 2021
Approval Letter: Approval Order
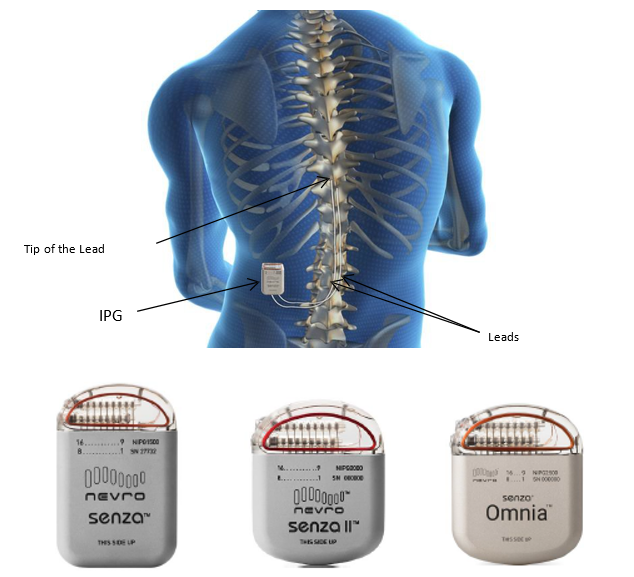
What is it? The Senza Spinal Cord Stimulation (SCS) system is an implanted, rechargeable spinal cord stimulation system intended to treat long-term (chronic) pain in the trunk or limbs that is difficult to manage (intractable).
The main components of the SCS system include an implanted signal generator that is connected to one or two implanted leads and a remote control that can turn the stimulator ON or OFF to allow adjustments of therapy settings.
This supplement expands the Senza SCS systems’ indications for use to include lower limb pain associated with diabetic neuropathy, also known as painful diabetic neuropathy.
How does it work? The implanted signal generator receives radio signals from the remote control which tell the signal generator when to deliver appropriate stimulation to the spinal cord. The external remote control is battery operated and can be controlled by the patient or a health care provider.
When is it used? The SCS system is used as an aid to manage chronic pain in the trunk or limbs, including one-sided or two-sided pain associated with failed back surgery syndrome, intractable low back pain, leg pain, and pain associated with nerve damage caused by diabetes (diabetic neuropathy).
What will it accomplish? The SCS system may help treat chronic, intractable pain in a patient’s trunk or limbs and pain associated with diabetic neuropathy.
When should it not be used? The SCS system should not be used in patients who:
- Cannot operate the SCS system
- Have not received effective pain relief during trial stimulation
- Are poor SCS surgical candidates
Additional information (including warnings, precautions, and adverse events):