Resolute Onyx Zotarolimus-Eluting Coronary Stent System, Onyx Frontier Zotarolimus-Eluting Coronary Stent System – P160043/S058
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Names: Resolute Onyx Zotarolimus-Eluting Coronary Stent System (Resolute Onyx), Onyx Frontier Zotarolimus-Eluting Coronary Stent System (Onyx Frontier)
PMA Applicant: Medtronic Vascular
Address: 3576 Unocal Place Santa Rosa, CA 95403
Approval Date: September 15, 2022
Approval Letter: Approval Order
What is it?
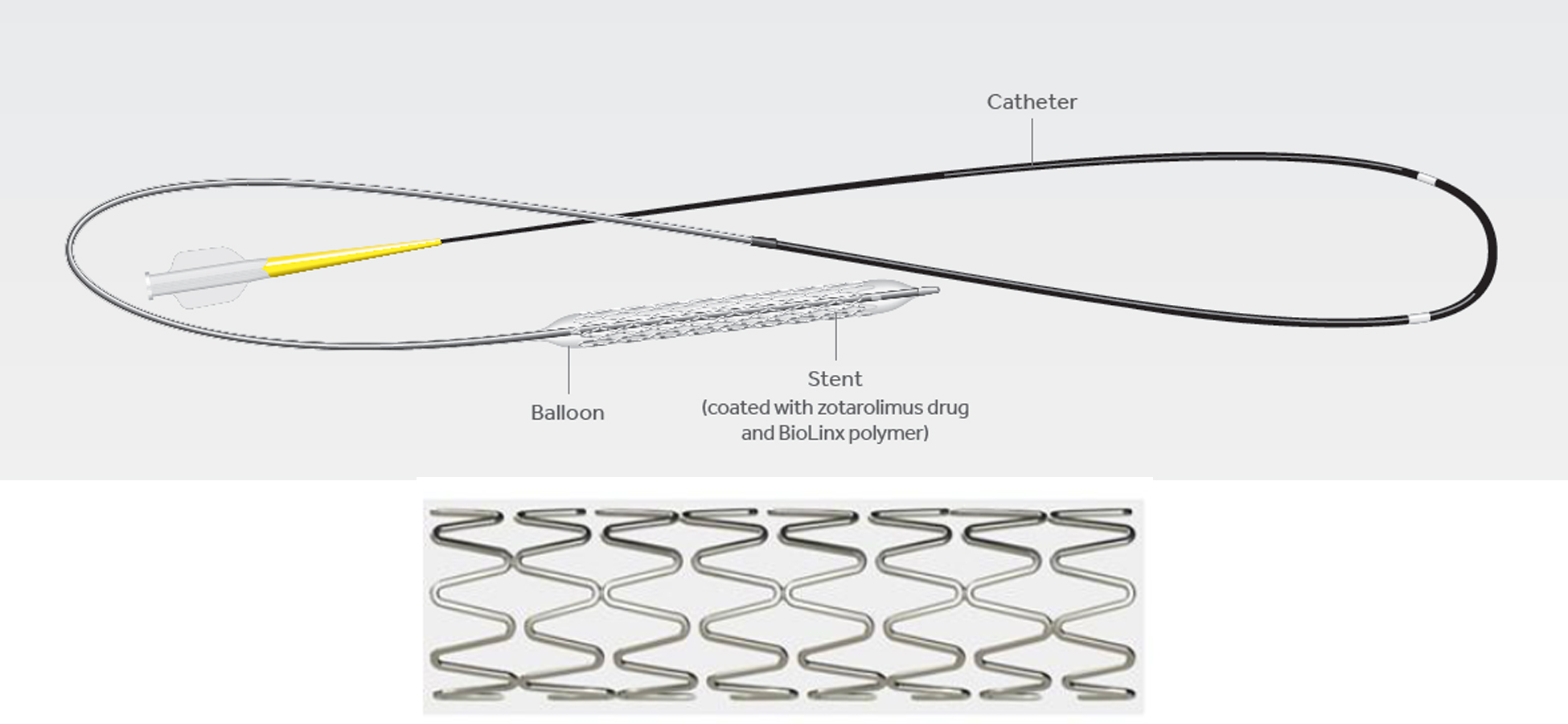
The Resolute Onyx Zotarolimus-Eluting Coronary Stent System (Resolute Onyx) and Onyx Frontier Zotarolimus-Eluting Coronary Stent System (Onyx Frontier) are intended to treat a narrowed blood vessel (coronary artery) caused by coronary artery disease. Both systems consist of a metal stent, made of a cobalt alloy surrounding an inner core made of platinum/iridium alloy, and a catheter delivery system. The stent is coated with the drug zotarolimus and a polymer (BioLinx) coating to help deliver the drug at a controlled rate.
This approval expands the indications for use to include certain bifurcation lesions, which are a type of coronary artery disease in a blood vessel that intersects a smaller blood vessel, or side branch.
How does it work?
A doctor inserts the Resolute Onyx or Onyx Frontier delivery balloon catheter into a blood vessel in the patient’s arm or groin. The stent is then positioned at the site of the coronary artery and the balloon is inflated. This expands the stent and presses it against the coronary artery wall. The stent remains permanently implanted within the coronary artery to help keep it open and improve the supply of blood and oxygen to the heart. The drug (zotarolimus) is released over time from the Onyx stent surface into the coronary artery wall to help prevent re-narrowing of the coronary artery, which sometimes occurs after a stent is implanted.
When is it used?
The Resolute Onyx and Onyx Frontier stent systems can be used in people who have a narrowing in their coronary arteries that involves two intersecting blood vessels (bifurcation lesion). The lesion should not involve the left main coronary artery (non-left main), and the planned treatment should be to place the stent in the main vessel, not the side branch (provisional stenting technique).
What will it accomplish?
In the primary clinical study, 205 patients with bifurcation lesions were treated using one of the Onyx stent systems. After one year, the results showed that people with non-left main bifurcation lesions treated using a provisional stenting strategy had a combined rate of 6.9% for death, heart attack, and need for additional procedures. This rate was better than the performance goal set before beginning the trial.
When should it not be used?
Resolute Onyx and Onyx Frontier stent systems should not be used in people with known hypersensitivity or allergy to:
- Aspirin, heparin, bivalirudin, clopidogrel, prasugrel, ticagrelor, ticlopidine, drugs such as zotarolimus, tacrolimus, sirolimus, everolimus, or similar drugs or any other analogue or derivative
- The cobalt-based alloy (cobalt, nickel, chromium, and molybdenum) or platinum-iridium alloy
- The BioLinx polymer or its individual components
Coronary artery stenting should not be used for people who:
- Cannot have anti-platelet and/or anticoagulation therapy.
- Have a lesion that prevents complete inflation of an angioplasty balloon or proper placement of the stent or stent delivery system.