REFLECT Scoliosis Correction System – H210002
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Probable Benefit and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: REFLECT Scoliosis Correction System
HDE Applicant: Globus Medical, Inc.
Address: Valley Forge Business Center
2560 General Armistead Avenue, Audubon, PA 19403
Approval Date: May 15, 2023
Approval Letter: Approval Order
What is it?
The REFLECT Scoliosis Correction System is a non-fusion spinal device intended to treat idiopathic scoliosis, an abnormal curve of the spine that happens without a known cause, in children and adolescents whose bones have not fully matured.
How does it work?
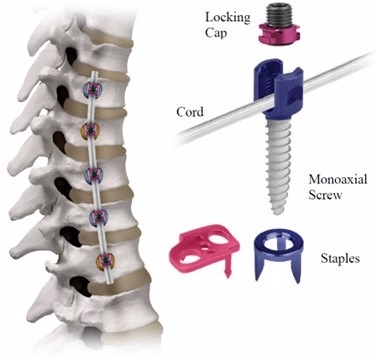
The REFLECT™ Scoliosis Correction System includes bone screws, staples, a cord, and locking caps. Staples and bone screws are placed into the spine on the side of the spinal curve during orthopedic surgery. The cord is attached to the bone screws using locking caps. The surgeon applies tension to the cord to partially straighten the curved spine. After surgery, the cord continues to straighten the spine as the person continues to grow.
When is it used?
The REFLECT Scoliosis Correction System is indicated for people whose bones have not stopped growing (skeletally immature) and who require surgery to obtain and maintain correction of their progressive idiopathic scoliosis because use of a brace to correct their spinal curvature was not successful (failed bracing or intolerant to brace wear).
What will it accomplish?
The clinical data suggests the REFLECT Scoliosis Correction System provides probable benefit to prevent spinal curve progression and avoid spinal fusion, the current standard-of-care treatment. The rate of additional surgeries (revisions and reoperations) reported for people treated with the REFLECT Scoliosis Correction System were greater when compared with spinal fusion. However, only three out of 20 patients required converting treatment to spinal fusion. Patient preference was positive for the REFLECT Scoliosis Correction System.
When should it not be used?
People with the following conditions should not be treated with the REFLECT Scoliosis Correction System:
- Any type of infection, or if the skin on the back or sides of the ribs and stomach is irritated, cut, or damaged.
- Previous surgery at the spinal levels where the scoliosis curve is located.
- Soft or less dense bone, or measured T-score (bone density measurement) of -1.5 or less.
- Skeletally mature with no spinal growth remaining.
- Any other medical or surgical condition that would not allow spinal surgery, such as:
- Problems with blood flow (too much or too little).
- Allergies to implant materials.
- Unwillingness or unable to follow doctor instructions after surgery.
Additional information (including warnings, precautions, and adverse events):