Prometra Programmable Infusion Pump System – P080012/S068
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Prometra Programmable Infusion Pump System
PMA Applicant: Flowonix Medical, Inc.
Address: 500 International Drive, Suite 200 Mount, Olive, NJ 07828
Approval Date: January 12, 2022
Approval Letter: Approval order
What is it?
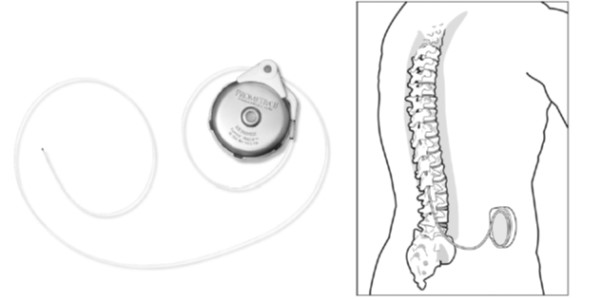
The Prometra® Programmable Infusion Pump System is a permanently implanted battery-operated programmable infusion pump that delivers medicine directly into the fluid-filled space around the spinal cord, called the intrathecal space.
The Prometra® Programmable Infusion Pump System includes a pump, programmer and catheter.
The Prometra® Pump System was previously approved for the intrathecal infusion of Infumorph, 0.9% Saline, and intrathecal Baclofen in adult patients. This approval expands indications for use to include the intrathecal infusion of Baclofen in patients ages 12 and older.
How does it work?
The infusion pump and catheter are both placed under the skin during surgery. The pump is usually placed at about waist level on one side of the abdomen, between the hip bone and the ribs. The catheter is threaded up the spine to the intrathecal space. After it is implanted, the pump is filled and programmed by a doctor using a remote control. The pump can be programmed to deliver medicine at a constant or variable rate. It can also be set to give a dosage of medicine repeated at specified times.
When is it used?
The Prometra® Programmable Infusion Pump System is used to deliver the drug Baclofen (baclofen injection, intrathecal, 500-2000 mcg/mL) into the spinal fluid (intrathecal) in patients 12 years of age and older.
What will it accomplish?
The Prometra® Programmable Infusion Pump System is intended to provide patients with continuous or chronic intrathecal infusions to treat muscle stiffness and pain that has not improved using other treatments. In clinical studies, the Prometra®Programmable Infusion Pump System has been shown to improve pain level and help reduce related disabilities from a baseline level in the first 6 months after it is implanted.
When should it not be used?
The Prometra® Programmable Infusion Pump System should not be used in patients with:
- An infection, such as a tooth abscess or a bed sore.
- A body type that cannot comfortably or safely accommodate the pump size and weight.
- An inability to have the pump implanted 2.5 cm (1 inch) under the skin.
- Allergies to the catheter materials, including silicone elastomers, barium sulfate, tungsten, polyacetal resin, ink, stainless steel, hydroglide hydro gel coating, or plastic needle hubs (polypropylene and acrylic based).
- Allergies to the pump materials, including titanium, silicone elastomers, polyphenylsulfone, silicone adhesive, polyvinylidene fluoride, MP35N metal (nickel-cobalt-chromium-molybdenum alloy), or stainless steel (AL29-4, 316L).
- A prior intolerance to implanted devices.
- Spinal column structure that stops cerebrospinal fluid from flowing freely or will not allow for intrathecal medicine delivery.
- The determination that they are not a suitable candidate based on psychological evaluation.
- Any reason where Infumorph or baclofen injection (intrathecal) cannot be used.