Perclose ProStyle Suture-Mediated Closure and Repair System and Perclose ProGlide Suture-Mediated Closure Systems – P960043/S118
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: Perclose ProStyle Suture-Mediated Closure and Repair System and
Perclose ProGlide Suture-Mediated Closure System
PMA Applicant: Abbott Vascular
Address: 3200 Lakeside Drive, Santa Clara, CA 95054
Approval Date: June 2, 2023
Approval Letter: Approval Order
What is it?
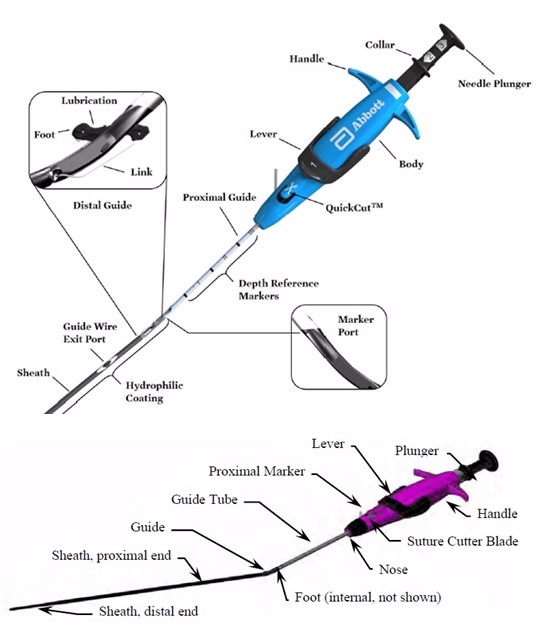
The Perclose ProStyle Suture-Mediated Closure and Repair System (ProStyle) and Perclose ProGlide Suture-Mediated Closure System (ProGlide) use a stitch (suture) to close openings made in blood vessels (access sites) during catheterization procedures. The suture stays in place after the procedure to prevent bleeding until the opening heals. ProStyle is a newer variation of the ProGlide device but is very similar. Both devices work in the same way.
This approval expands the indications for use to include closing multiple access sites in a single large blood vessel of the groin (femoral vein).
How does it work?
The ProGlide and ProStyle use one or two sutures to seal the opening created by a catheter at access sites in a femoral vein, either with or without additional pressure applied to stop access site bleeding. The suture is placed by inserting the end of the device into the vessel after the catheters are removed. A plunger and lever on the handle of the device move the suture and create a stitch across the access site.
When is it used?
The ProGlide and ProStyle are used to close the access site after the catheter is removed during a catheterization procedure. Both ProGlide and ProStyle can be used to close multiple access sites on the femoral veins.
What will it accomplish?
Clinical studies--including one primary study with 36 participants and two real-world doctor-led studies that included 647. In all the studies, people who had femoral vein access sites closed using ProGlide or ProStyle showed that people (fewer than 4 out of 100) had major bleeding or damage to the vein requiring surgery as result of using the device.
When should it not be used?
There are no known reasons not to use the ProGlide or ProStyle.
Additional information (including warnings, precautions, and adverse events):
- Summary of Safety and Effectiveness Data (SSED)
- Labeling
- Labeling 2 (Patient Guide)
- PMA Database Entry