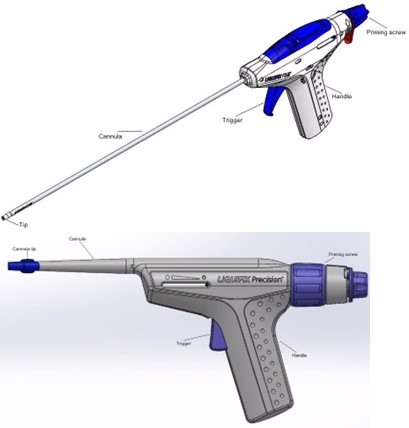
LiquiFix FIX8 and LiquiFix Precision Hernia Mesh Fixation Devices – P220024
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: LiquiFix FIX8 and LiquiFix Precision Hernia Mesh Fixation Devices
PMA Applicant: Advanced Medical Solutions Limited
Address: Western Wood Way, Langage Science Park
Plymouth, Devon PL7 5BG
United Kingdom
Approval Date: June 2, 2023
Approval Letter: Approval Order
What is it?
LiquiFix Hernia Mesh Fixation devices include a liquid adhesive and an applicator to apply it. These devices are used during surgery to repair groin (inguinal) and upper thigh (femoral) hernias.
How does it work?
The Liquifix FIX8 and Liquifix Precision Open Hernia Mesh Fixation applicator is inserted into the body where it is used to place drops of liquid adhesive on implanted hernia repair mesh attaching it to the underlying tissue. The Liquifix FIX8 Hernia Mesh Fixation device may also be used to re-connect the tissue lining the abdominal wall (peritoneum) over the implanted mesh. The adhesive remains in the body with the hernia mesh after hernia repair surgery is complete.
When is it used?
Liquifix FIX8 Hernia Mesh Fixation is used during laparoscopic, or minimally invasive, surgical repair of inguinal and femoral hernias. LIQUIFIX Precision Open Hernia Mesh Fixation is used during open surgical repair of inguinal and femoral hernias.
What will it accomplish?
A clinical study with 284 participants from five institutions showed that 142 people who had hernia repair surgery using Liquifix FIX8 reported similar levels of pain after surgery to the 142 people who had surgery using another approved product. The advantage of fixating the mesh with the LiquiFix adhesive rather than the control tacks was that it provided a broader point of fixation, could be applied more rapidly than suture and its application area was not restricted by anatomic structures such as blood vessels and nerves.
When should it not be used?
Liquifix Hernia Mesh Fixation devices should not be used with people who:
- Are not able to have prosthetic material, like implantable mesh, used in repair.
- Have hypersensitivity or allergy to cyanoacrylate adhesives, formaldehyde, or D&C Violet No. 2 dye.
Additionally, Liquifix Hernia Mesh Fixation should not be used to:
- Attach mesh made with polytetrafluoroethylene (PTFE) or anything that is not polypropylene or polyester.
- Close or fix brain (cerebral) tissue, blood vessels, or peripheral nerves.
Additional information (including warnings, precautions, and adverse events):