Inspire Upper Airway Stimulation - P130008/S089
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
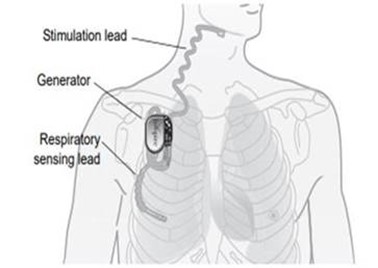
Product Name: Inspire Upper Airway Stimulation (UAS)
PMA Applicant: Inspire Medical Systems, Inc.
Address: 5500 Wayzata Blvd. Suite 1600, Golden Valley, MN 55416 US
Approval Date: March 20, 2023
Approval Letter: Approval Order
What is it?
The Inspire Upper Airway Stimulation (UAS) system is an implantable nerve stimulator used to treat obstructive sleep apnea. The Inspire UAS system includes the implantable pulse generator (IPG), stimulation lead, and sensing lead, as well as external components: the physician programmer and the patient remote.
How does it work?
The IPG detects the patient’s breathing pattern and maintains an open airway by stimulating the nerves using small electrical impulses. The doctor can program stimulation settings using the external physician programmer. The patient remote allows the user to turn on the treatment before falling asleep and turn it off when awake.
When is it used?
This approval is for the Inspire UAS system to be used for a subset of teenagers with Down syndrome who are between the ages of 13 and 18 with severe obstructive sleep apnea (as determined by an apnea-hypopnea index (AHI) of greater than or equal to 10 and less than or equal to 50). These patients must also:
- Not have complete blockage or concentric collapse of the back, muscular part of the roof of the mouth, called the soft palate.
- Not be a candidate for surgery to remove the adenoids and tonsils (adenotonsillectomy).
- Not be able to use or tolerate positive airway pressure (PAP) treatments after several tries.
- Have been considered for all other alternative/additional treatments that are considered the standard of care for this condition.
Inspire UAS was previously approved (P130008 and P130008/S039) in patients 18 years of age and older with moderate to severe obstructive sleep apnea (15≤AHI ≤65) who have been confirmed to fail or cannot tolerate PAP treatments including continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP), and who do not have a complete blockage of the upper airway.
What will it accomplish?
In a clinical study of 42 young people with Down syndrome and obstructive sleep apnea, Inspire UAS treatments gave the majority of patients a clinically meaningful reduction in the severity of their obstructive sleep apnea and improved their quality of life. At a 12-month follow up, more than half (65.9%) of patients had at least a 50% reduction in their AHI score and 73.2% reported less than 10 sleep apnea events per hour. That means they moved from the severe obstructive sleep apnea category to a lower level of severity. Quality of life as assessed by the Epworth Sleepiness Scale (ESS) showed improvement as well, with an average reduction of 5.1 points and a change in the average ESS score from a baseline of 10.0 to 5.0.
These findings, along with what was learned from existing clinical data taken from an adult trial, STAR, along with real-world evidence from the ADHERE registry, supported expanding the indications to include this new population.
When should it not be used?
The Inspire UAS system should not be used when:
- Other types of apnea (central and mixed) besides obstructive sleep apnea make up more than 25% of the total AHI score.
- There is a physical condition that would keep upper airway stimulation from working well, such as a complete blockage of the upper airway.
- Any condition or previous treatment will compromise or prevent neurological control of the upper airway.
- Patients cannot operate the sleep remote or do not have necessary assistance to operate it.
- Patients are pregnant or plan to become pregnant.
- A patient has another implantable device that could possibly have an unintended interaction with the Inspire system. Contact the device manufacturer to assess the possibility of interaction.
- The patient requires magnetic resonance imaging (MRI) other than what is specified in the MR Conditional labeling for this system.
Additional information (including warnings, precautions, and adverse events):