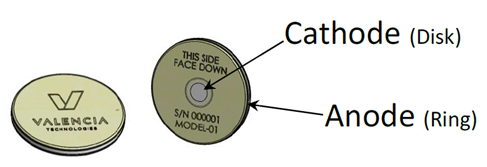
eCoin Peripheral Neurostimulator – P200036
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: eCoin® Peripheral Neurostimulator
Applicant: Valencia Technologies Corporation
Address: 28464 Westinghouse Place Valencia, CA 91355
Approval Date: March 1, 2022
Approval Letter: Approval order
What is it?
The eCoin Peripheral Neurostimulator System generates electrical pulses to help stimulate nerves related to bladder control in people with urgency urinary incontinence, or a sudden urge to urinate that causes some urine to leak out. The device is implanted under the skin near the ankle and is controlled by a healthcare provider using a remote control.
How does it work?
The eCoin Peripheral Neurostimulator System is implanted under the skin of the ankle to stimulate the tibial nerve, a nerve that allows feeling and movement to parts of the leg and foot. This nerve also has some influence over the nerves that control the bladder. Once implanted, the eCoin system delivers electrical pulses to the tibial nerve in 30-minute sessions based on a fixed schedule. A healthcare provider can adjust the level of stimulation based on each patient’s sensitivity and needs, using the remote control.
Nerves controlling the pelvic organs, including the bladder, originate from the lower spine and are connected to the tibial nerve in the leg, though it is not known exactly how neurostimulation of the tibial nerve helps stimulate the nerves controlling the bladder.
When is it used?
The eCoin Peripheral Neurostimulator System is intended for use in patients who have urgency urinary incontinence and who have not had success at treating their condition through behavior changes such as pelvic exercises, limiting fluid intake, scheduling times to urinate or through medications.
What will it accomplish?
In a clinical study, 68% of patients (90/132) with urgency urinary incontinence had a 50% or greater reduction in urgency urinary incontinence episodes up to 48 months after activating the eCoin Peripheral Neurostimulator System.
Valencia Technologies Corporation will also conduct a 5-year Post-Approval Study (PAS) to study real-world effectiveness of this system for treatment of urgency urinary incontinence. The study will also record any related adverse events of interest.
When should it not be used?
The eCoin Peripheral Neurostimulator System should not be used in people have:
- A history of surgery in the implant area of the ankle
- Previous, unhealed damage or infections near the implant area
- Lower leg or foot conditions, including:
- Open wounds or sores on the lower leg or foot
- Swelling from excess fluid build-up in the lower leg
- Vein or artery disease or insufficiency in the lower leg
- Inflammation or skin conditions in the lower leg
Additionally, the system should not be used in patients who are unable to operate the device’s Patient Controller Magnet.