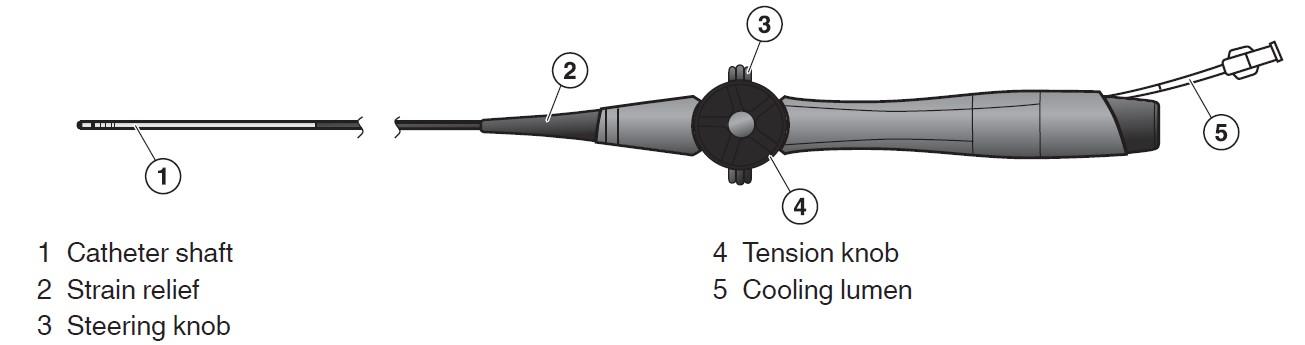
DiamondTemp Ablation Catheters - P200028
This is a brief overview of information related to FDA’s approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval.
Product Name: DiamondTempTM Ablation Catheter
PMA Applicant: Medtronic Inc.
Address: 8200 Coral Sea Street NE, Mounds View, MN 55112 USA
Approval Date: January 28, 2021
Approval Letter: Approval Order
What is it?
The DiamondTemp Ablation Catheters are used to treat a certain kind of abnormal heart rhythm (arrhythmia) called paroxysmal atrial fibrillation, by destroying (ablating) a small area of the heart tissue.
How does it work?
A doctor inserts the catheter into a blood vessel (vein) in the groin and advances it to reach the heart. Once inside the heart, doctors use the catheter to identify the areas of the heart that cause the abnormal heart rhythm. The tip of the catheter then delivers heat energy to destroy the areas of heart tissue that are causing the abnormal heart rhythm. The doctor removes the catheter after treatment.
When is it used?
The DiamondTempTM Ablation Catheters are used to treat paroxysmal atrial fibrillation, when medication does not work well or cannot be tolerated due to side effects.
What will it accomplish?
The DiamondTempTM Ablation Catheters is intended to prevent episodes of rapid heartbeat or other symptoms due to atrial fibrillation. In a clinical study, 239 patients with paroxysmal atrial fibrillation were treated by the DiamondTemp™ Ablation Catheters. At the completion of treatment, the abnormal heart rhythm was successfully corrected in 228 patients (95.4%). At one year after the treatment, the abnormal heart rhythm remained corrected in 142 patients (59.4%) without medication. In 189 patients (79.1%), the abnormal heart rhythm remained corrected with or without medication at one year after treatment.
When should it not be used?
The DiamondTempTM Ablation Catheters should not be used in:
- Patients with an ongoing infection in the bloodstream
- Patients with an artificial heart valve
- Patients who have a tumor or blood clot inside the heart
- Patients who have had a medical device placed in the heart to close a hole in the wall between the two upper chambers of the heart)
- Patients unable to receive heparin (a blood thinner) or an acceptable alternative medicine to prevent blood clotting
- Pregnant women
- Patients who are younger than 18 years of age
- Patients with unstable blood pressure