The Arctic Front Advance and Arctic Front Advance Pro and The Freezor MAX Cardiac Cryoablation Catheters – P100010/S110
This is a brief overview of information related to FDA’s approval to market this product for expanded use. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA’s approval of a new use.
Product Name:
Arctic Front Advance Cardiac Cryoablation Catheters
Arctic Front Advance Pro Cardiac Cryoablation Catheters
Freezor MAX Cardiac Cryoablation Catheter
CryoConsole
Manual Retraction Kit
PMA Applicant: Medtronic Inc.
Address: 8200 Coral Sea Street N.E., MVS46, Mounds View, MN 55112
Approval Date: June 18, 2021
Approval Letter: Approval Order
What is it?
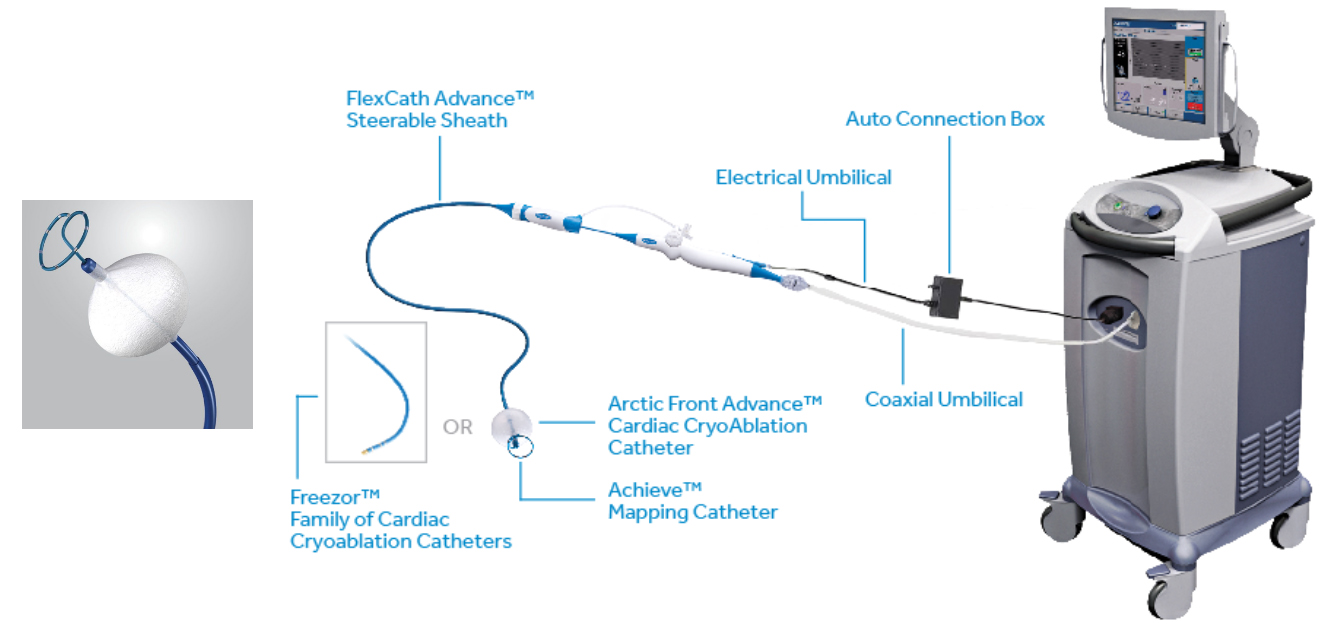
The Arctic Front Advance and Arctic Front Advance Pro Cardiac Cryoablation Catheters are balloon-tipped catheters that use cold energy to treat an abnormal heart rhythm that causes fast and irregular heartbeats (atrial fibrillation). The Freezor MAX Cardiac Cryoablation Catheter is a secondary catheter that uses the same cold energy to treat parts of the heart that the balloon-tipped catheter cannot reach.
This approval expands the indications for use to also treat patients with symptomatic paroxysmal AF who have not been previously treated with medicine (drug naïve) for preventing AF episodes from occurring again. The FDA previously approved these catheters to treat patients with intermittent AF episodes (paroxysmal AF) or continuous AF episodes (persistent AF) lasting up to six months in duration that cause symptoms (symptomatic) and do not respond to medicine (drug refractory).
How does it work?
A doctor puts the balloon-tipped catheter through a small cut in a vein in the groin and moves the tip of the catheter up to the heart. The balloon is then inflated at the opening of a vein that carries blood from the lung to the heart (pulmonary vein). The doctor sends gas into the balloon to make it very cold. The cold balloon is used to freeze (ablate) the heart tissue and block electrical signals that cause abnormal heart rhythms. This is repeated to treat all the pulmonary veins. The Freezor MAX catheter ablates areas of the heart tissue that are not reached by the balloon-tipped catheter. Both catheters are removed after treatment.
When is it used?
A doctor uses these catheters to treat patients who have drug refractory symptomatic paroxysmal AF or persistent AF of less than six months in duration to prevent AF episodes from occurring again. These catheters may now be used as an alternative to medicine for initial treatment of recurrent symptomatic paroxysmal AF.
What will it accomplish?
In a clinical study, 203 patients with paroxysmal AF who had not previously received treatment with medicine were selected to participate. Of the patients selected, 104 patients received treatment with these catheters and 99 patients received treatment with medicine. Every patient was followed for one year. Of the 104 patients treated with these catheters, 73.7% did not have a return of an abnormal heart rhythm. Of the 99 patients treated with medicine, 45% did not have a return of an abnormal heart rhythm.
When should it not be used?
The catheters should not be used in patients who:
- Have infections in their bloodstreams
- Have tumors or clots inside their hearts
- Have a small mesh tube (called a stent) placed in the pulmonary vein
- Have a blood thickening disorder called cryoglobulinemia