Magnetic Field Interference with Programmable CSF Shunts
Patients implanted with magnetic externally programmable CSF shunt valves may have a risk of experiencing an unintended change in their valve setting when exposed to strong magnetic fields from external magnetic sources and implanted medical devices that use magnets. The health consequences of unintended changes to CSF shunt valve settings include symptoms that, if unchecked, may become serious.
The FDA encourages health care providers to report all possible cases of unintended valve changes through the FDA MedWatch Program or through the FDA Medical Product Safety Network (MedSun) if your facility is an active participant in the MedSun program.
- The FDA believes all marketed CSF shunt systems are safe and effective when used as intended.
- The FDA does not currently advise patients with magnetic externally programmable CSF shunt valves to avoid using specific electronic devices.
Magnetic Interference
The FDA conducted (between January 1998 and October 2012) an analysis about the possible interaction between programmable CSF shunt valves and external sources containing magnets such as cell phones, electronic tablet devices, cordless power drills, headphones and earbuds. The FDA also conducted (2019) an analysis regarding the possible interaction between programmable CSF shunt valves and implanted hearing devices containing magnets, since these devices are in close proximity to one other. Patients implanted with magnetic externally programmable CSF shunt valves may have a risk of experiencing an unintended change in their valve setting when exposed to strong magnetic fields.
While there is a risk of unintended changes in a programmable CSF shunt valve setting due to magnet interference, the prevalence is unknown. Patients should be aware that magnets may affect valve settings in magnetic externally programmable CSF shunt systems.
The FDA studied commonly used magnets and their field strengths to understand if and how they may affect magnetic externally programmable CSF shunt valves. The studies demonstrated that the unintended change of the CSF shunt system rapidly diminishes the farther away the magnetic source is from the CSF shunt. Although the FDA's findings were not comprehensive, they can be used as a basis for suggested safe distances between magnetic externally programmable CSF shunt valves and magnetic sources. Implanted medical devices that use magnets were not included in this study. In the case of implanted hearing devices that contain magnets, health care providers should follow the recommendations listed in our Programmable CSF Shunts and Magnetic Field Interference with Implanted Hearing Devices Letter to Health Care Providers.
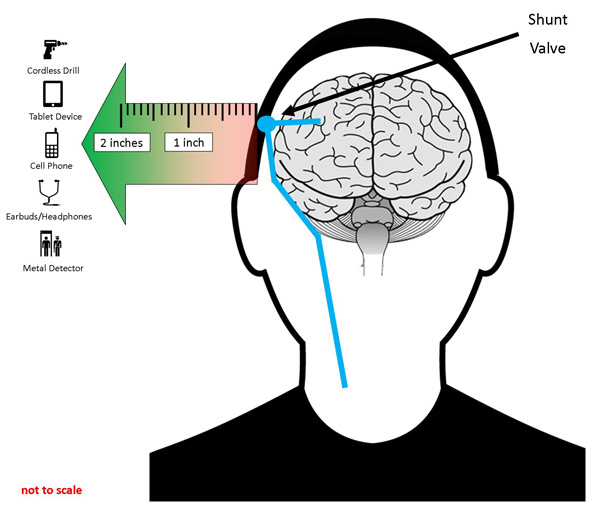
Figure 1 illustrates several common magnetic sources. The FDA's studies have shown that these sources are safe if a distance of two inches or more is maintained between the magnet and the site of the implanted shunt valve, although differences in body physiology and magnet characteristics may affect the results.
Figure 1: The FDA suggests keeping products that contain magnets two or more inches away from the location of magnetic externally programmable CSF shunt valves. Patients are advised to use the ear opposite the shunt for devices requiring listening (such as cell phones and earbuds).
For implanted hearing devices that use magnets, health care providers should follow the recommendations listed in our Programmable CSF Shunts and Magnetic Field Interference with Implanted Hearing Devices Letter to Health Care Providers.