ESG Next Generation
ESG NextGen Background
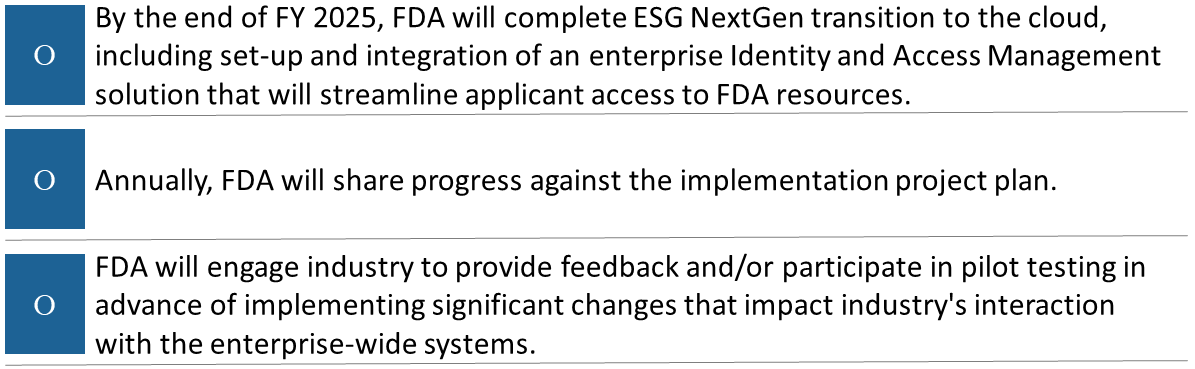
As part of the FY2025 PDUFA commitments, the FDA will modernize the electronic submission gateway (ESG) by implementing an improved cloud-based modernization architecture that leverages an enterprise Identity and Access Management solution and that supports greatly expanding data submission bandwidth and storage, while continuing to ensure its stable operation. The modernization of the electronic submission gateway is the Electronic Submission Gateway Next Generation (ESG NextGen).
ESG NextGen Vision
The FDA plans to modernize the ESG to take advantage of latest cloud technology and best-of-breed COTS software. The ESG cloud modernization, along with additional analysis and enhancements, is a part of an initiative known as the ESG NextGen solution. The vision of ESG NextGen, as illustrated in the figure below, is to provide the FDA with a trusted, secure cloud-based, and unified intake gateway that is highly available, scalable, and accepts a variety of electronic submissions for processing. The ESG NextGen will be capable of accepting all electronic submissions from industry, sponsors, manufacturers, applicants, and other governmental agencies.
To provide feedback, please email EsgNgSupport@fda.hhs.gov