FDA shows generic lamotrigine extended-release tablets are bioequivalent to innovator drug in fully replicated crossover bioequivalence study
CDER researchers conducted a study of the antiepileptic drug, lamotrigine, comparing generic lamotrigine extended-release (ER) tablets and brand ER tablets in healthy subjects. Their finding that the generic and brand tablets were bioequivalent supports the use of generic-brand substitution of lamotrigine ER products for patients.
Lamotrigine is one of the most prescribed antiepileptic (AED) drug among available AEDs,1 given its broad-spectrum efficacy and good tolerability.2,3 U.S Food and Drug Administration (FDA)-approved oral formulations of lamotrigine generics include immediate-release (IR), extended-release (ER), chewable, and orally disintegrating tablets.4 Previous FDA-funded clinical studies (i.e., EQUIGENExternal Link Disclaimer and BEEPExternal Link Disclaimer) demonstrated bioequivalence (BE) for generic lamotrigine IR products in epilepsy patients with generic substitution. However, due to the more complex mechanism of controlled-release systems compared to the IR formulations, concerns about the risk of generic-brand substitution of lamotrigine ER products have remained within medical community and patient populations, so further study was conducted.5
Addressing the concerns
To address concerns considering the possible changes in pharmacokinetic (PK) profile shapes, PK variations associated with possible within-subject variability (WSV), the FDA-study evaluated; (1) the pharmacokinetics and BE of generic and brand lamotrigine ER products in a fully replicated BE study6 in healthy subjects, and (2) whether such fully replicated study design and WSV data could better support the approval of generic lamotrigine ER products. The choice of a single dose study in healthy subjects was based on the findings from the previous EQUIGEN and BEEP studies that BE studies performed in patients with epilepsy receiving multiple dosing showed comparable findings to single dose studies in healthy subjects, along with that the healthy subjects are more homogenous and more sensitive to detect formulation differences. Additionally, FDA conducted this clinical study to evaluate a generic lamotrigine ER product approved prior to 2016 at a strength (200 mg) that was not tested in vivo. This study is conducted at fed state as the generic lamotrigine ER product is predicted to have the most disparate PK profile from brand under fed condition (i.e., shorter Tmax).
The first open-label, single-dose, two-treatment, four-period, two-sequence, fully replicated crossover BE study compared generic lamotrigine ER tablet to brand Lamictal XR (200 mg) in 30 healthy subjects under fed conditions. Pharmacokinetics (PK) profiles were generated based on intensive blood samplings. An additional in silico simulation study focusing on formulation differences, in vivo PK profile at steady state as well as incorporating WSV,7,8 helped confirm BE and offered further insights on brand-to-generic lamotrigine ER switching.
Discussion
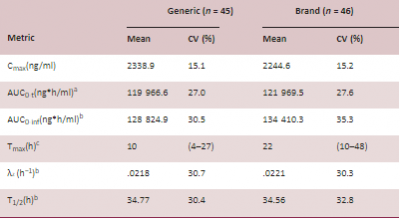
The generic and brand lamotrigine ER products showed comparable peak serum concentration as well as overall exposure in the study subjects (Table 1). Similar to what was observed in the PK BE studies at the 50 mg strength submitted to support the approved original abbreviated new drug application (ANDA), the median time to reach maximum drug concentration (Tmax) for the generic and brand products, respectively, indicated that the generic product has a faster release rate compared to the brand product.
TABLE 1 Summary of pharmacokinetics (PK) metrics for generic and brand lamotrigine extended-release (ER) tablets
Note: The total number of subjects receiving each treatment in four periods were 45 for generic (Test) and 46 for brand (Reference). A subject receiving repeated treatments in two periods will have PK data of both periods.
aGeneric n = 44, brand n = 45.
bGeneric n = 43, brand n = 44.
cMedian and range.
Patients typically take lamotrigine every day long term and build up a stable drug concentration level. The generic drug product will provide the same long term clinical benefit if they can build similar stable steady state levels. To assess the impact of the shifted Tmax on the steady state PK following chronic dosing of the drug products, a BE analysis was conducted with simulated PK data following repeated dosing of the 200 mg generic and brand products. All subjects achieved the steady state of drug concentration by the fifth week of the study. In Figure 1(A), the brand name and generic products have comparable (differences within 10%)maximum drug concentrations (Cmax) and minimum drug concentrations (Cmin) at steady state, respectively. The geometric mean ratio of PK metrics comparing the generic to brand name products fall within the 80-125% limit, implying lack of clinical significance.
To further assess the potential PK differences after the brand-to-generic switch, subjects on the 200 mg brand product for 5 weeks (34 days) were switched to the generic product on Day 35. In the switch scenario [Figure 1(B)], there is only a difference on Cmax on the first day immediately after switch, while other PK parameters including AUC and Cmin are similar. The difference on Cmax diminishes after that. All PK metrics met the 80-125% BE criteria after Day 1 (Table 2). The transient and marginal Cmax variation on the first day after switch is considered clinically insignificant given the chronic use of this drug, and the safety margin of lamotrigine as compared to other AED drugs with narrow therapeutic index.
Figure 1 (A) Simulated PK profiles after repeated dosing in the fifth week. Brand (Reference) and generic (Test) are presented in red and blue, respectively.
The solid lines are the mean plasma concentrations, whereas the shaded area represent the 95% confidence intervals (CIs) of the simulated plasma concentrations.
(B) Simulated PK profiles after a brand-to-generic switch on Day 35. Brand and brand-to-generic switch are presented in red and blue, respectively. The solid lines are the mean plasma concentrations, whereas the shaded area represent the 95% confidence intervals (CIs) of the simulated plasma concentrations.
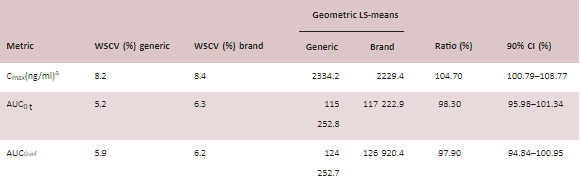
TABLE 2 Summary of the bioequivalence (BE) analysis results for generic and brand lamotrigine extended-release (ER) tablets.
Abbreviation: WSCV, within-subject coefficient of variation.
aGeneric n = 45, brand n = 46.
bGeneric n = 44, brand n = 45.
cGeneric n = 43, brand n = 44.
Conclusion
This FDA study has demonstrated the BE of generic lamotrigine ER tablets, and the brand ER tablets (200 mg) in healthy subjects under fed conditions using a randomized, single dose, fully replicated crossover study. Single doses of both generic and brand lamotrigine ER tablet showed similar WSVs and were safe and well tolerated in the healthy subjects. The generic and brand ER drug products are expected to be bioequivalent following a switch and chronic use of the generic, as shown by the in silico simulated PK data following repeated dosing of 200 mg lamotrigine ER tablets.
Such a finding, added to findings from previous studies of generic lamotrigine IR products, further supports the generic-brand substitution of the generic lamotrigine oral products. Although lamotrigine is not considered a narrow therapeutic index (NTI) drug, the WSVs of both generic and brand products are small and fall well within the FDA standards for NTI drugs. The study results supported the appropriateness of a two-way, cross-over study design to demonstrate BE and generic-brand substitution of lamotrigine ER products.
How this research improves generic drug development and supports patient access to needed drugs
Competition among generic drug makers with their brand name counterparts has helped make drugs in the U.S. more widely available and generally less expensive, helping patients better access needed medicines. The U.S. Food and Drug Administration (FDA) recently conducted a new study to address concerns about the risks of generic-brand substitution of lamotrigine, a commonly prescribed antiepileptic drug, extended-release (ER) tablets for the brand tablets, taking into consideration the complexity of controlled release systems and pharmacokinetic variations associated with possible within-subject variability. The generic lamotrigine ER tablets demonstrated bioequivalence (BE) to the brand product in a fully replicated BE study with healthy subjects, supporting the generic-brand substitution of lamotrigine ER tablets.
(This Impact Story is based on the article “Generic lamotrigine extended-release tablets are bioequivalent to innovator drug in fully replicated crossover bioequivalence study,” published in the Journal Epilepsia, on October 13, 2022, and authored by Lanyan Fang, Zhichuan Li, Minori Kinjo, Sara Lomonaco, Nan Zheng, Wenlei Jiang, and Liang Zhao.9)
REFERENCES/NOTES
1Vossler DG, Weingarten M, Gidal BE. Summary of antiepileptic drugs available in The United States of America: working to- ward a world without epilepsy. Epilepsy Curr. 2018;18:1–26.
2Yasam VR, Jakki SL, Senthil V, Eswaramoorthy M, Shanmuganathan S, Arjunan K, et al. A pharmacological over- view of lamotrigine for the treatment of epilepsy. Expert Rev Clin Pharmacol. 2016;9:1533–46.
3Werz MA. Pharmacotherapeutics of epilepsy: use of lamotrig- ine and expectations for lamotrigine extended-release. Ther Clin Risk Manag. 2008;4:1035–46.
4U.S. Food and Drug Administration. Approved drug prod- ucts with therapeutic equivalence evaluation. 42nd Edition. Available at: https://www.fda.gov/media/71474/download. Accessed 09/22/2022.
5Johnson EL, Chang YT, Davit B, Gidal BE, Krauss GL. Assessing bioequivalence of generic modified-release antiepileptic drugs. Neurology. 2016 Apr 26;86(17):1597-604. doi: 10.1212/WNL.0000000000002607. Epub 2016 Mar 25. Erratum in: Neurology. 2016 Jul 26;87(4):447. PMID: 27016518; PMCID: PMC4844239. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4844239/
6A fully replicated BE study is a 2-sequence, 2-treatment, 4-period, crossover BE study (sequence 1: TRTR, and sequence 2: RTRT) in which all subjects receive two repeated treatments of T and R in different periods, respectively.
7Li Z, Fang L, Jiang W, Kim MJ, Zhao L. Risk-based bioequiv- alence recommendations for antiepileptic drugs. Curr Neurol Neurosci Rep. 2017;17:82.
8Johnson EL, Chang YT, Davit B, Gidal BE, Krauss GL. Assessing bioequivalence of generic modified-release antiepileptic drugs. Neurology. 2016;86:1597–604.
9Fang L, Li Z, Kinjo M, Lomonaco S, Zheng N, Jiang W, et al. Generic lamotrigine extended-release tablets are bioequivalent to innovator drug in fully replicated crossover bioequivalence study. Epilepsia. 2023;64:152–161. https://doi.org/10.1111/epi.17438External Link Disclaimer